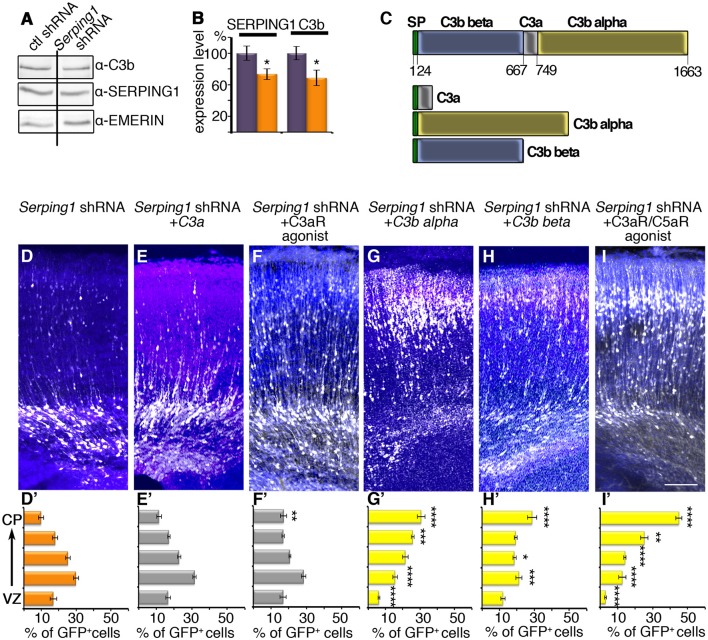
Figure 6.
Serping1 in the complement pathway. (A,B) Levels of activated C3 (C3b) are slightly reduced in the Serping1 shRNA compared to control. Areas from the brains electroporated in utero on E14 with control or Serping1 shRNA were dissected on E17 and subjected to western blot analysis with anti-C3b antibodies, anti-SERPING1 and anti-EMERIN antibodies. The levels of SERPING1 and C3b were normalized to EMERIN and quantified (n = 9). *p < 0.05. (C) Schematic representation of C3. The proteolytic cleavage of C3 on aa 667 and aa 749 results in C3a peptide and C3b, composed of two subunits: C3b beta and C3b alpha. Three fragments mimicking the cleavage products of C3, all with an N-terminal signal peptide (SP, green). (D–I) Rescue of Serping1 shRNA migration phenotype with C3 fragments and peptides, agonists of Complement receptors. Brains were electroporated (E14-E18) with Serping1 shRNA alone (D) or together with either C3a (E), C3aR agonist (F), C3b alpha (G), C3b beta (H), or dual C3aR/C5aR agonist (I). The scale bar is 100 μm. The distribution of neurons along the cortex in 5 bins is shown (D'–I'). All conditions were compared to Serping1 shRNA. Two-way ANOVA, n = 11, *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001.