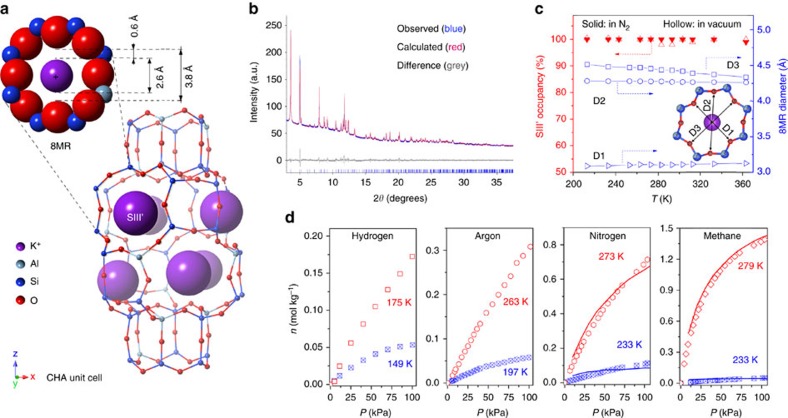
Figure 1. Paradox of non-polar gas molecule admission through the apparently blocked pores of a potassium chabazite.
(a) Illustration of the structure of an ideal potassium chabazite crystal, highlighting the eight-membered oxygen ring (8MR) which sets the nominal pore aperture diameter to be 3.8 Å (distance between opposing oxygen atoms). The centre of the 8MR pore aperture (cation site SIII′) is occupied by the potassium cation, reducing the nominal accessible pore aperture to 0.6 Å, as determined from the Synchrotron PXRD data. (b) Rietveld refinement of a representative Synchrotron PXRD pattern for trapdoor potassium chabazite with a Si/Al ratio of 2.2 (r2KCHA). (c) Left axis: fractional occupancy of SIII′ sites by K+ cations indicating blockage of 8MR pores over the experimental temperature range. Estimated uncertainty 1.5%. Right axis: evolution of the 8MR dimensions D1, D2 and D3 (inset) of r2KCHA suggesting pore contraction with increasing temperature, ruling out the possibility of thermally induced pore dilation. 0.3% uncertainty. (d) Adsorption isotherms for hydrogen, argon, nitrogen and methane, respectively, on r2KCHA showing restricted pore accessibility at low temperatures (crossed blue symbols) but no restriction at high temperatures (red open symbols), and comparison with GCMC calculated equilibrium capacities (lines) for nitrogen and methane.