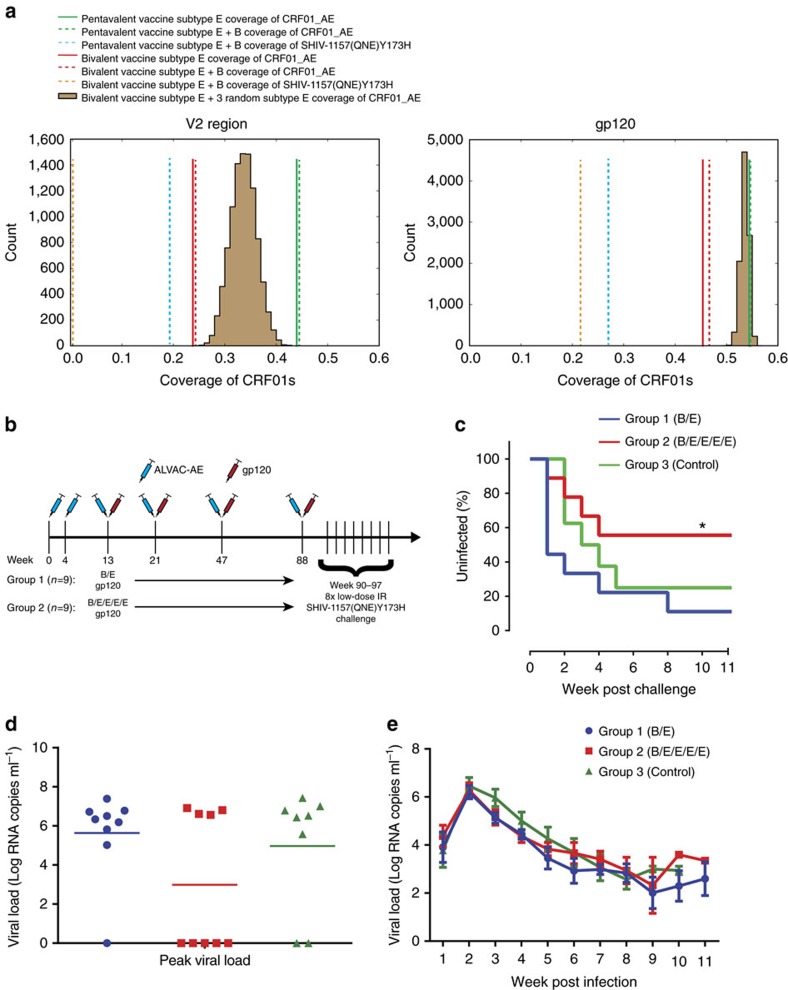
Figure 1. Pentavalent vaccine improved coverage of HIV-1 diversity and had increased protection from SHIV challenge.
(a) The sequence coverage of the RV144 viral V2 epitope region (HXB2 positions 154–184) and full subtype E gp120 by the pentavalent vaccine subtype Es (green line; 92TH023, A244, AA058, AA104 and AA107), the pentavalent vaccine subtype Es and B (63521; dashed green line), the pentavalent subtype E and B coverage of the challenge SHIV-1157(QNE)Y173H (dashed blue line), the RV144 bivalent subtype Es (92TH023 and A244; red line), the RV144 bivalent subtypes E and B (63521; dashed red line) and the RV144 bivalent vaccine subtype E plus B SHIV coverage (dashed orange line). The distribution of sequence coverage of 10,000 randomly selected sets of three clade E viruses when combined with A244 and 92TH023 (brown). (b) Schematic of the immunization and challenge regimen. Eighteen rhesus macaques are administered two doses of ALVAC-AE, and then animals either received ALVAC-AE plus a bivalent (n=9) or pentavalent (n=9) protein boost four times. Then all animals were subjected to 8 weekly low-dose intrarectal challenges with SHIV-1157(QNE)Y173H. Unimmunized animals (n=8) were challenged as the control arm. (c) KM plot showing the percentage of uninfected animals after 8 weekly challenges (*Group 2 vs Group 1, P=0.02; Group 2 vs Group 3, P=0.48; one-tailed KM log-rank test). (d) Peak viral load of the infected animals from each vaccine and control group. (e) Viral load tested weekly after initial infection in all the groups. Lines are group means and error bars indicate s.e.m.