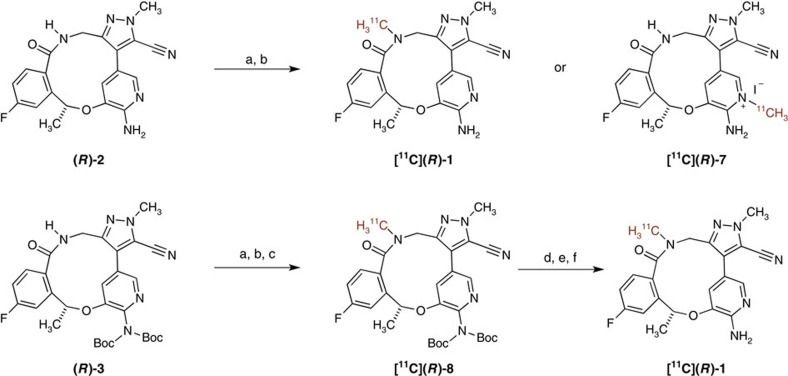
Figure 2. Development of [11C]lorlatinib.
The one-step reaction (top) presented challenging separation of the precursor (R)-2 from [11C]lorlatinib and confirmation of identity, which is likely attributed to [11C](R)-7. A two-step methodology (bottom) allowed for facile removal of the precursor and simplified the automation for routine production. Reagents and conditions: (a) 1 mg of precursor ((R)-2 or (R)-3), 0.7 eq TBAOH (1 M in methanol), in 80 μl anhydrous DMF; (b) [11C]CH3I, via ‘Loop Method'; (c) HPLC purification (see ESI); (d) 0.7 ml of 6 M HCl, 80 °C, 5 min; (e) 4.5 ml of 1 N NaOH, 6 ml of 3 M NaOAc, 6 ml H2O; (f) SPE reformulation.