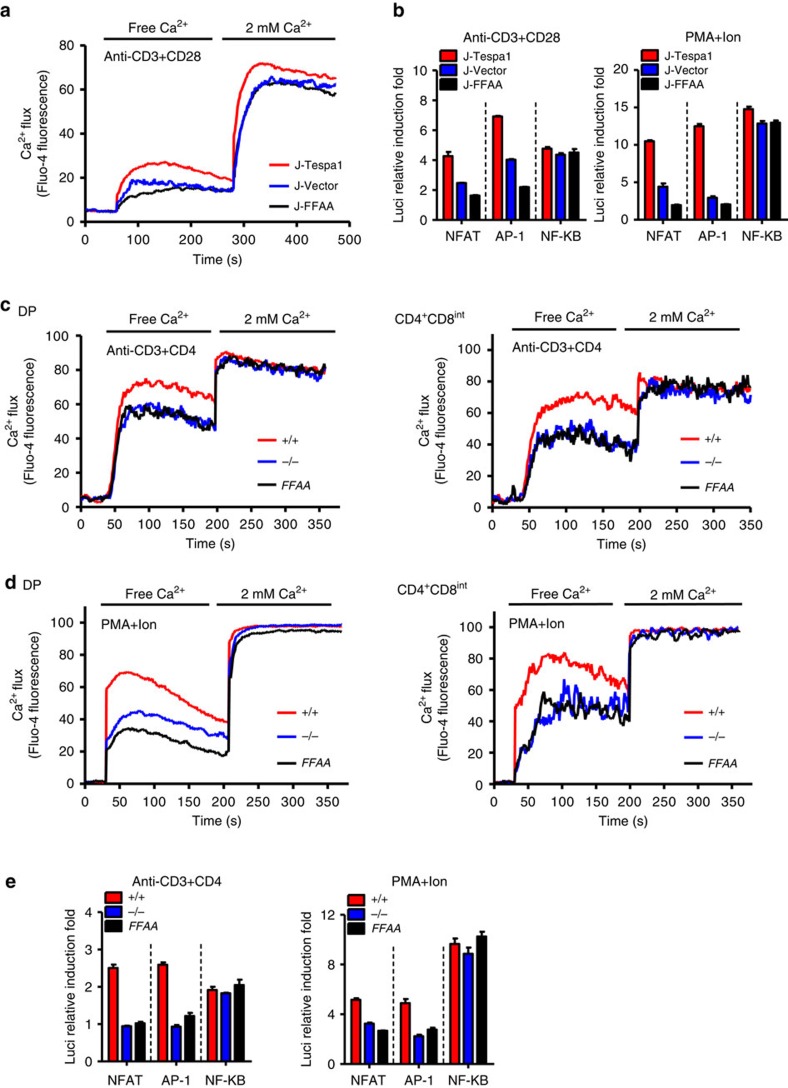
Figure 5. Tespa1-IP3R1 interaction facilitates calcium signalling.
(a) Recordings of Ca2+ flux in J-Tespa1, J-FFAA, and J-Vector cells. Cells were stimulated by crosslinking biotinylated anti-CD3 and anti-CD28 antibodies (anti-CD3+CD28) with streptavidin in Ca2+-free medium, then, 2 mM CaCl2 was added. (b) Luciferase activity in J-Tespa1, J-FFAA and J-Vector cells transfected with luciferase reporter constructs (horizontal line). Cells were allowed to ‘rest’ for 30 min, and were then stimulated with anti-CD3 plus anti-CD28 antibodies (left) or PMA plus ionomycin (right) for 6 h, results were normalized to renilla luciferase activity and are presented relative to those of unstimulated cells. (c,d) Recordings of Ca2+ flux from Tespa1+/+ (+/+), Tespa1−/− (−/−) and Tespa1-FFAA (FFAA) thymocytes. Cells were stimulated by crosslinking biotinylated anti-CD3 and anti-CD4 antibodies (anti-CD3+CD4, c) with streptavidin or by PMA and ionomycin (PMA+Ion, d) in Ca2+-free medium, then, 2 mM CaCl2 was added. (e) Luciferase activity in sorted DP thymocytes from Tespa1+/+ (+/+), Tespa1−/− (−/−) and Tespa1-FFAA (FFAA) mice. Cells were transfected with luciferase reporter constructs (horizontal line), allowed to ‘rest’ for 30 min, and then stimulated with anti-CD3 and anti-CD4 antibodies (left) or PMA plus ionomycin (right) for 6 h, results were normalized to renilla luciferase activity and are presented relative to those of unstimulated cells. Data are representative of at least three experiments (error bars indicate s.d. in b,e).