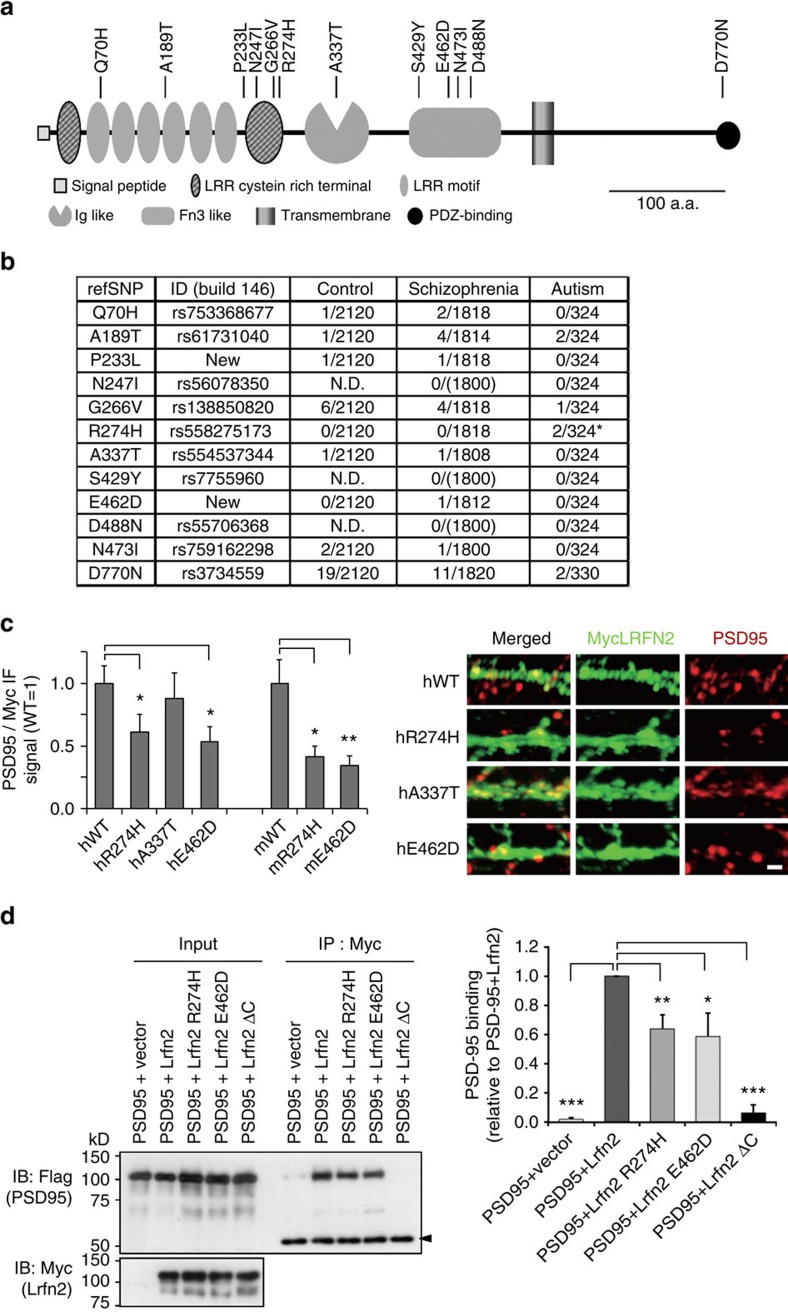
Figure 9. Resequencing analysis of the LRFN2 protein-coding region from Asian patients with autism or schizophrenia.
(a) Nonsynonymous SNPs on a schematic of the LRFN2 molecule. (b) Nonsynonymous SNPs in human LRFN2 from patients with schizophrenia or autism and healthy controls. refSNP ID, the numbers indicated those deposited in the NCBI database and ‘new' indicates the newly identified nonsynonymous mutations in this study. The numbers of control, schizophrenia and autism columns indicate (allele number of patients with the mutations)/(allele number of total patients), where 0/(1,800) means the absence of the patients with corresponding mutations in the schizophrenia patients groups (n=1,820). Ambiguously genotyped subjects were excluded. Note that all carriers with mutations were heterozygotes. N.D., not determined. *P<0.05, Fisher's exact test. (c) Effects of the identified mutations of the subcellular localization of human LRFN2 (h) and mouse Lrfn2 (m) proteins. Co-localized PSD-95 signals (red) on those of Myc-tagged WT proteins or mutant proteins (green) with PSD-95 were quantified in cultured hippocampal neurons. Values for WT were adjusted to one (n=42–45 neurons from 15 images for each group; *P<0.05, **P<0.01, compared to WT, t-test). Similar results were obtained in three independent experiments. Right panels indicate the representative images (scale bar, 5 μm). Those for mouse proteins are indicated in Supplementary Fig. 8d. (d) PSD-95-binding capacities of WT and mutant mouse Lrfn2 proteins. Myc-tagged Lrfn2 proteins including Lrfn2ΔC were co-expressed with Flag-tagged PSD-95 in COS7 cells. Immunopreciptation was carried out using anti-Myc epitope antibody. Co-precipitated Flag-PSD-95 was detected with anti-Flag epitope antibody. Right graph indicates the densitometric quantification of the immunoblot (n=8 independent experiments; *P<0.05, **P<0.01, ***P<0.001, compared to WT, t-test).