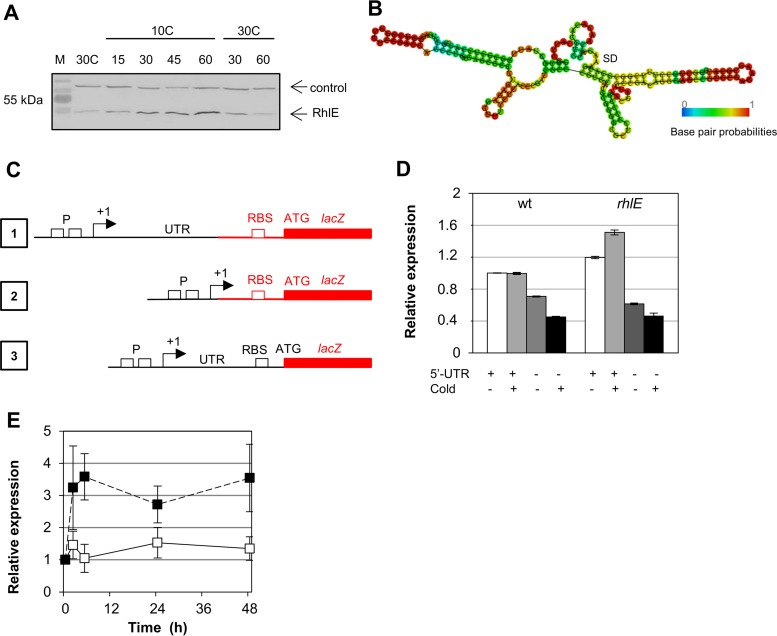
FIG 4.
Regulation of RhlE in response to low temperature. (A) C. crescentus strain MM84, containing a tagged FLAG-RhlE protein, was incubated at 30°C to mid-log phase and then transferred to 10°C for 1 h. After this time, the culture was returned to 30°C for 2 h. Aliquots were taken at the indicated times, and protein accumulation was evaluated sequentially by immunoblotting using an anti-FLAG antibody and the anti-Fur antiserum. The arrows indicate the RhlE protein and a nonspecific protein that reacts with the anti-Fur serum as a control. M, prestained molecular mass marker. (B) Minimal free energy prediction of secondary structure of the rhlE 5′ UTR. (C) Schematic representation of the transcriptional and translational fusions to lacZ: 1, transcriptional fusion in pRKlacZ290 containing the promoter region (P) and the 5′ UTR; 2, transcriptional fusion in pRKlacZ290 containing only the promoter region; 3, translational fusion in pJBZ281 containing the promoter region, the 5′ UTR, the ribosome binding site (RBS), and the first codon of RhlE fused to lacZ in frame. In black are the elements that came from the rhlE locus, and in red are the vector sequences. (D) Expression of transcriptional fusions of the rhlE promoter in the presence or absence of the 5′ UTR to the lacZ reporter gene. Cultures were grown at 30°C to mid-log phase and then incubated at 10°C for 6 h, and expression was determined by β-galactosidase activity assays at these times. Results shown are the means of results from two experiments, and the range is indicated by vertical bars. (E) C. crescentus NA1000 harboring a translational fusion of the rhlE promoter and 5′ UTR to the lacZ reporter gene was grown at 30°C up to mid-log phase. Culture samples continued to grow at 30°C (white squares) or were incubated at 10°C (black squares), and expression was determined by β-galactosidase activity assays at the times indicated. Results shown are the means of results from two experiments, and standard deviation is indicated by vertical bars.