FIG 7.
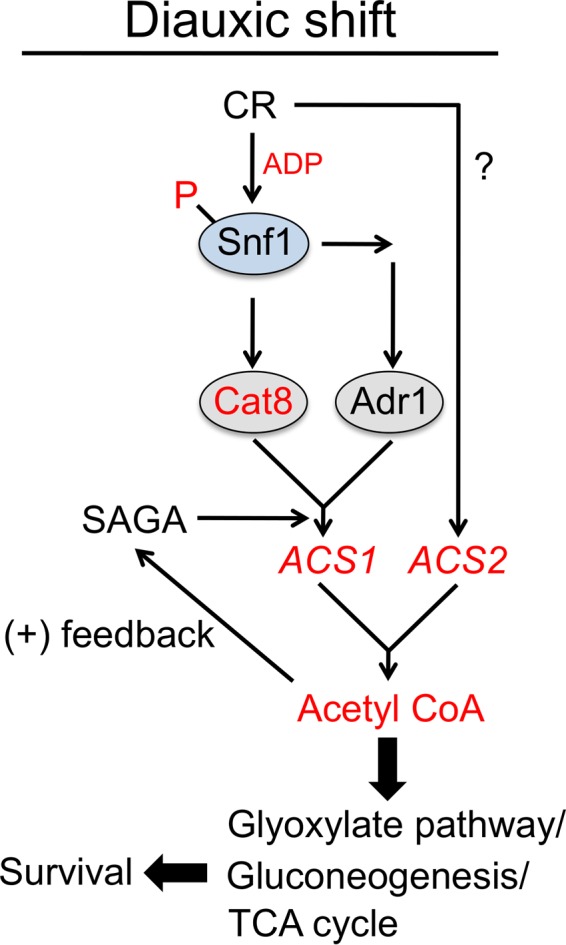
Model for CR-induced optimization of acetyl-CoA production during the diauxic shift to extend yeast CLS. CR maximizes Snf1 T210 phosphorylation in part by maintaining elevated ADP levels. Snf1 activates Cat8 and Adr1 transcription factors, which turn on multiple genes, including the acetyl-CoA synthetase gene ACS1. CR also maintains high ACS2 expression during the diauxic shift but independently of Cat8/Adr1. Elevated nucleocytoplasmic acetyl-CoA promotes histone acetyltransferase activity of the SAGA transcriptional coactivator complex, which amplifies expression of ACS1 and a subset of other Cat8/Adr1 target genes, resulting in a positive-feedback loop that drives sufficient acetyl-CoA production. Carbon skeletons are shunted through the glyoxylate cycle and gluconeogenesis pathway, supporting biomass formation during the early stages of the diauxic shift and storage of carbohydrates for extended cell survival. The full TCA cycle and respiration become more important later. Circles indicate proteins, and italics indicate genes. Red indicates components upregulated or enriched by CR (compared to NR) during or after the diauxic shift.
