Abstract
Background
Vascular pathology is common in late-life depression (LLD) and may contribute to alterations in cerebral blood flow (CBF) and cerebrovascular reactivity (CVR). In turn, such hemodynamic deficits may adversely affect brain function and clinical course. The goal of this study was to examine whether altered cerebral hemodynamics in depressed elders predicted antidepressant response.
Methods
21 depressed elders completed cranial 3T MRI, including a pseudo-continuous Arterial Spin Labeling (pcASL) acquisition on both room air and during a hypercapnia challenge. Participants then completed 12 weeks of open-label sertraline. Statistical analyses examined the relationship between regional normalized CBF and CVR values and change in Montgomery-Asberg Depression Rating Scale (MADRS) and tested for differences based on remission status.
Results
10 participants remitted and 11 did not. After controlling for age and baseline MADRS, greater change in MADRS with treatment was associated with lower pre-treatment normalized CBF in the caudal anterior cingulate cortex (cACC) and lateral orbitofrontal cortex (OFC), as well as lower CVR with hypercapnia in the caudal medial frontal gyrus (cMFG). After controlling for age and baseline MADRS score, remitters exhibited lower CBF in the cACC and lower CVR in the cMFG.
Limitations
Our sample was small, did not include a placebo arm, and we examined only specific regions of interest.
Conclusions
Our findings suggest that increased perfusion of the OFC and the ACC is associated with a poor antidepressant response. They do not support that vascular pathology as measured by CBF and CVR negatively affects acute treatment outcomes.
Keywords: Depression, aging, geriatrics, perfusion, MRI, ssri
1. Introduction
Late life depression (LLD), or Major Depressive Disorder occurring in individuals over age 60 years, is a heterogeneous disorder with a community prevalence of approximately 4–5% (Taylor 2014a; Park et al. 2015). It is more common in medically ill populations and is associated with greater medical comorbidity, including higher rates of vascular risk factors (Taylor et al. 2004, Colloby et al. 2012). Such observations contributed to the ‘vascular depression’ hypothesis (Alexopoulos et al. 1997) that proposed “cerebrovascular disease may predispose, precipitate and perpetuate some geriatric depressive syndromes.” This theory led to substantial research in LLD investigating the influence of vascular disease on the occurrence and outcomes of depression (Taylor et al. 2013). However, despite a large body of research, the mechanisms by which sub-ischemic vascular disease may influence the presentation or course of depression remain unclear.
The “Hypoperfusion Hypothesis,” proposes that reductions in cerebral perfusion negatively affect brain circuits involved in cognition and mood regulation, and so influence the presentation of depression (Taylor et al. 2013). Illnesses common in LLD, such as hypertension, diabetes, and atherosclerosis, contribute to vascular pathology by causing vascular wall hypertrophy, endothelial cell dysfunction, and reduced arterial diameter and distensibility (de la Torre 2012; Dandona et al. 2004;). This vascular pathology is often greater in individuals with LLD (Paranthaman et al. 2010; Greenstein et al. 2010) and negatively affects the cerebral vasculature’s autoregulatory processes (Direk et al. 2012; Tiemeier 2002), resulting in decreased cerebral blood flow (CBF; ml/100g/min) in brain regions supplied by affected vessels. When severe, such hypoperfusion may lead to ischemia but even milder deficits in CBF may negatively affect protein synthesis required for neuronal function (Kleim et al. 2003; Debiec et al. 2002;). In turn, these deficits may contribute to abnormal cognitive function (Heo et al. 2010; Rabbitt et al. 2006).
Past work supports that depression is associated with altered CBF, although results are not always consistent across studies. Positron Emission Tomography (PET) and Single Photon Emission Computed Tomography (SPECT) measures of blood flow demonstrate global and regional changes in CBF in individuals with major depression, including differences in the prefrontal cortex, the anterior cingulate cortex (ACC), temporal, and parietal regions. Studies of unmedicated depressed adults demonstrate reduced CBF in the middle and inferior frontal gyri and the right anterior cingulate gyrus (Monkul et al. 2012), while studies of antidepressant non-responders found higher CBF in the prefrontal and cingulate cortices (Brockmann et al. 2009) and higher CBF in middle temporal regions (Videbech et al. 2001). CBF in different brain regions may also be related to particular depressive symptoms. For example, subgenual cingulate cortex CBF is inversely correlated with insomnia while orbitofrontal cortex CBF is positively correlated with anxiety (Perico et al. 2005). Importantly, the majority of studies focus on younger or midlife adult populations rather than specifically on older adults.
MRI techniques such as Arterial Spin Labeling (ASL) also measures CBF. ASL MRI holds several advantages over PET and SPECT techniques, as it is safe and non-invasive with no ionizing radiation or exogenous contrast requirement. ASL uses arterial water magnetization as an endogenous tracer and measures CBF by subtracting the signal from consecutively acquired images with and without magnetic arterial blood water labeling (Williams et al. 1992). The use of ASL to study depression is limited and, as seen with PET and SPECT studies of perfusion, results have been inconsistent. In depressed adult populations, significant reductions in CBF are reported in the ACC and inferior prefrontal cortex (Ota et al. 2014, Lui et al. 2009) while treatment-resistant patients exhibit increased CBF in the prefrontal cortex and subgenual and rostral ACC (Duhameau et al. 2010). A study in depressed adolescents identified both regional increases and decreases in CBF, finding reduced CBF in the inferior frontal gyrus and dorsolateral prefrontal cortex, but increased CBF in the subcallosal cingulate gyrus (Ho et al. 2013). Finally, CBF differences in depression may not be limited to gray matter, as LLD is associated with increased CBF in subcortical white matter (Colloby et al. 2012).
CBF alone does not provide a complete picture of dynamic vascular processes. Cerebrovascular reactivity (CVR) is a measure of the vasculature’s ability to modify CBF in response to metabolic or vascular demands and can be measured experimentally using a pharmacological challenge with acetazolamide (Noguchi et al. 2011) or more recently, a mild non-invasive hypercapnic challenge where levels of inhaled carbon dioxide are increased (Zaharchuk et al. 1999; Donahue et al. 2014). Vascular pathology may thus not only contribute to a reduction in basal CBF, but could also impair the ability of the vasculature to respond to increased metabolic needs if the parenchyma is operating near autoregulatory capacity or if the mechanisms of vasoreactivity are themselves impaired (Glodzik et al. 2011). CVR to hypercapnia decreases with age and is impaired in individuals with vascular disease or increased cerebrovascular risk factor severity (Glodzik et al. 2011; Hajjar et al. 2010; Lu et al. 2011). Such CVR findings may have clinical significance as reduced CVR may predict cognitive decline (Silverstrini et al. 2006).
The aim of the current study was to determine whether baseline measures of CBF and CVR are predictive of antidepressant response in older depressed adults. Based on our hypoperfusion hypothesis, we hypothesized that poor response to a 12-week open-label trial of sertraline would be associated with lower frontocingulate CBF and less CVR in response to a hypercapnia challenge. We also hypothesized that remitters will exhibit higher frontocingulate baseline CBF than non-remitters. However, as studies in other age groups have associated increased CBF with depression (Chi et al. 2015) we also examined whether increased CBF was associated with clinical outcomes.
2. Methods
2.1. Design and sample
The study included twenty-one depressed subjects aged 60 years or older who met DSM-IV-TR criteria for Major Depressive Disorder. The Mini-International Neuropsychiatric Interview (MINI) (Sheehan et al. 1998) assessment was administered at the start of the study to determine psychiatric diagnoses and the results were confirmed by a clinical interview conducted by a geriatric psychiatrist (WDT). At study entry, participants had to have Montgomery-Asberg Depression Rating Scale (MADRS) (Montgomery and Asberg 1979) score of 15 or more and a Mini-Mental Status Exam (MMSE) (Folstein et al. 1975) of 22 or more.
Exclusion criteria included (1) current or past diagnoses of other Axis I psychiatric disorders, including panic disorder and substance dependence; (2) any use of illicit substances (such as marijuana or cocaine) or abuse of prescription medications (such as benzodiazepines or opiates) within the last three months ; (3) presence of acute suicidality; (4) current or past psychosis; (5) primary neurologic disorders, including dementia or history of stroke or transient ischemic attacks; (6) chronic untreated medical disorders where treatment was warranted; (7) need for continuous oxygen use or any medical disorder where the hypercapnia challenge would put the subject at increased risk; and (8) contraindications for magnetic resonance imaging (MRI). Other exclusion criteria pertaining to antidepressant treatment included (1) receiving ECT in last 6 months; (2) use of antidepressant medications in the last month (or the last 6 weeks for fluoxetine); (3) known allergy or hypersensitivity to sertraline; (4) a failed therapeutic trial of sertraline in the current depressive episode; and (5) current or planned psychotherapy.
Participants were recruited from community advertisements and clinical referrals to the Vanderbilt University Medical Center Psychiatry Outpatient Clinics. The Vanderbilt Institutional Review Board approved the study and all participants provided written informed consent.
2.2. Assessments, antidepressant treatment and monitoring
At baseline, participants provided demographic data and completed screening assessments including the MINI and the MMSE. As part of their clinical interview, the study psychiatrist evaluated depression severity with the MADRS, assessed medical comorbidity using the Cumulative Illness Rating Scale for Geriatrics (CIRS-G) (Miller et al. 1992), and quantified vascular risk using the Framingham Study stroke risk prediction tool (Wolf et al. 1991).
Participants who met entry criteria but were currently taking antidepressant medications had those medications tapered and discontinued. They were antidepressant-free for at least two weeks prior to MRI. A minority of depressed participants took sedatives as needed for sleep at bedtime, either zolpidem (5–10mg, N=4) or lorazepam (1mg, N=1). For these individuals, MRI was scheduled in the early afternoon to assure there were no residual drug effects from the night before.
After completing cranial MRI, participants entered a 12-week open-label trial of sertraline. Sertraline was started at 25mg for two days, and then increased to 50mg daily. If tolerated and indicated by continuing depressive symptoms, the dose could be increased by 50mg every two weeks to a maximum dose of 200mg daily. For subjects who experienced problems with tolerability, reductions to the previous dose were allowed. Participants had study contacts every two weeks, via clinic visits every 4 weeks with scheduled telephone calls in the intervening 2 week intervals. At each contact, depression severity was measured via MADRS along with assessments for adverse events and medication compliance.
2.3. MRI Acquisition
All participants were scanned on the same research-dedicated 3.0T Philips Achieva whole-body scanner (Philips Medical Systems, Best, The Netherlands). All subjects completed ASL and structural imaging with data acquired using body coil radiofrequency transmission and a 32-channel head coil for reception. Structural imaging utilized a whole-brain T1-weighted MPRAGE image with TR/TE = 8.7/4.6 and spatial resolution = 0.9 x 0.9 x 1.2 mm, along with a FLAIR image for hyperintensity assessment, using TR = 10,000, TI = 2700, and TE = 125. During scan sessions participants wore a close-fitting oxygen facemask that covered the mouth and nose. Air mixtures were provided continuously at 12 L/min using this setup. Physiological monitoring during ASL sequences was achieved using an In Vivo Research Inc. device and Millenia Vital Systems Monitoring System (3155 MVS). This allowed for the measurement of arterial oxygen saturation fraction and exhaled end-tidal CO2 (EtCO2) using a nasal cannula throughout the gas delivery paradigms.
A pseudocontinuous ASL (pCASL) sequence was used for CBF and CVR assessments (representative images shown in Fig. 1). This utilized a 1.65s labeling pulse train consisting of Hanning-windowed 0.7ms pulses, followed by a post-labeling delay of 1.6s (TR=4s, TE=14ms; spatial resolution = 3 x 3 x 7 mm3). A separate equilibrium magnetization image was acquired with identical geometry but spin labeling preparation removed for M0 calculation. The entire sequence included 75 total dynamics alternating between normocapnia (room air) and hypercapnia. For CBF measures, participants received medical grade room air (approximately 20.9% O2 and 78.1% N2, with a balance of other trace atmospheric elements). These data were drawn only from the first four minutes (30 dynamics).
Fig. 1.
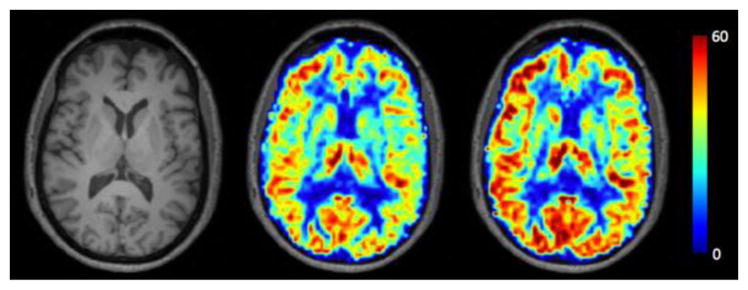
Example of CBF and CVR Images, Images from a representative subject displaying the T1-weighted image (left) with the corresponding normocapnia CBF image (center) and hypercapnia CBF image (right). Darker red colors indicate higher levels of CBF, while blue indicates low CBF. CBF scales between 0–60 ml/100g/min.
For CVR assessment, identical pCASL sequences were obtained during a hypercapnic normoxia challenge where participants inhaled a mixture of 5% CO2 with 21% O2 and 74% N2. To prevent prolonged continuous exposure to hypercapnic conditions and assure safety, this stimulus paradigm consisted of two blocks of hypercapnic normoxia (2 min; 15 dynamics each) interleaved with medical-grade room air (2 min;15 dynamics). The hypercapnic blocks were combined for analyses, while the interleaved room air block was discarded.
2.4. MRI Analyses
After correcting for motion using mcflirt (in the FSL package), ASL images were pairwise subtracted to get a difference in the magnetization image for each dynamic. These data were then averaged across time within each condition (medical air or hypercapnic normoxia) to consolidate the results and decrease noise. CBF was quantified via application of the flow-modified Bloch equation (Alsop et al. 2015) using a per-slice modified post-labeling delay (slicedt) of 23ms. The M0 image was registered to the average control image to allow M0 to be applied in a voxel-wise manner. As recommend by Alsop et al. (2015), we used a labeling efficiency of 0.85.
We utilized FreeSurfer tissue segmentation and cortical parcellation of the T1 data to obtain regions-of-interest limited to gray matter regions in the frontal lobe and cingulate cortex. FreeSurfer (version 5.1) analyses were conducted in a high-performance Linux cluster environment using standard previously published methods (Fischl 2004; Fischl et al. 2004). All FreeSurfer parcellations were inspected and manual edits performed as needed. To allow for comparison across subjects and utilize region-of-interest analyses, all ASL images were co-registered to the T1 images using FSL’s flirt with a mutual information cost function limited to six degrees of freedom. To avoid altering the ASL data, we then inverted that transform to register the T1 images into the ASL space and applied this transform to the FreeSurfer parcellation using nearest-neighbor interpolation. This allowed us to calculate the mean and median CBF value for each region of interest in each condition (medical air or hypercapnic normoxia). To eliminate outliers and the endovascular signal from the median and mean calculations, we only included voxels within a wide physiological CBF range between 1 and 100 ml/100g/min.
White matter hyperintensity (WMH) volumes, findings on T2-weighted or FLAIR images that are related to cerebral ischemia, were measured using the Lesion Segmentation Toolbox (Schmidt et al. 2012). This was implemented through the VBM8 toolbox in SPM8, using the default threshold of 0.3. In native space, each voxel on the T1 image is assigned as gray matter, white matter, or CSF. After bias-correction the FLAIR is coregistered to the T1 image. The toolbox initially creates a conservative binary WMH map based on outlier values across the T1 and FLAIR images. Next, a lesion-growth algorithm using Markov Random Fields modeling extends this conservative map to define the extent of the WMH. This lesion map is then used to calculate total cerebral WMH volume.
2.5. Statistical Analyses
All data analyses were conducted using SAS 9.4 (Cary, NC). Regional CBF measures utilized medical air ASL data only and were normalized for total cerebral perfusion, calculated as regional CBF divided by total cortical CBF. Regional CVR measures were calculated as the difference in CBF between hypercapnic normoxia and medical air conditions, divided by CBF on medical air. Based on our hypotheses, we focused on frontal lobe and cingulate lobe subregions as delineated by FreeSurfer segmentation.
Using these CBF and CVR measures, we examined three sets of potential relationships between ASL measures and depressive symptoms. As advanced age is associated with vascular risk factors, vascular pathology, and WMH severity, we included age as a covariate in all models. First, in models controlling for age, we examined the relationship of CBF and CVR with baseline depression severity measured by MADRS. Next, in models controlling for age and baseline MADRS score, we examined as the primary outcome measure, the relationship of CBF or CVR with change in depression severity over the course of the 12-week trial. As a secondary outcome measure, we tested for differences in CBF between individuals who did and did not remit over the course of study participation, defining remission as a final MADRS score of 7 or less. We similarly tested for differences based on response, defined as a 50% or greater improvement in MADRS score. For individuals who completed at least one follow-up assessment but withdrew early we used a last observation carried forward (LOCF) approach, wherein the last available MADRS score was used as the final score.
Finally, in post-hoc analyses, we examined the effect of WMH volumes on ASL measures and whether WMH measures altered the observed relationships between ASL measures and change in depression severity. For these analyses, we focused only on frontocingulate regions implicated in previous analyses. To examine total cerebral WMH effects on ASL measures, we examined CBF or CVR as dependent variables, with independent variables of age and WMH volume. To examine whether WMH volume altered previous findings, we added it as an additional independent variable to models examining change in depression severity while controlling for age and baseline MADRS score.
3. Results
Twenty-one eligible patients were enrolled in the study, took sertraline and completed at least one follow-up session. An additional 10 participants enrolled in the study but were withdrawn prior to receiving study medication as they did not meet entry criteria (2 due to concomitant medication issues, 4 did not meet the minimum depression severity criterion and 4 due to MRI contraindications). Using a definition of remission of achieving a final study MADRS of 7 or less, 10 participants remitted (R) and 11 did not (NR). There were no statistically significant differences between remitting and non-remitting subjects in demographic variables, baseline MADRS scores, MMSE score, or medical comorbidity severity, either globally measured by the CIRS or related to specific vascular risk measured by the Framingham Study stroke risk prediction tool or WMH volume (Table 1). The mean final daily dose of sertraline was 97.5 mg (SD=46.3mg, range 50–200mg) for remitting participants and 144.4mg (SD=58.3mg, range 50–200mg) for nonremitting participants (t = 1.95, 19df, p = 0.0675).
Table 1.
Differences between sertraline remitters and nonremitters
| Remitters (SD) (N=10) |
Nonremitters (SD) (N=11) |
t-value | p value | |
|---|---|---|---|---|
| Age | 69.1 (7.7) | 67.1 (6.9) | 0.63 | 0.5341 |
| Sex (% F) | 63.6 (7) | 60.0 (6) | - | 1.0000 |
| Education – yrs. | 15.9 (2.1) | 15.0 (1.6) | 1.13 | 0.2717 |
| Framingham | 9.7 (4.1) | 9.9 (3.7) | 0.12 | 0.9036 |
| CIRS | 4.5 (2.9) | 4.9 (2.8) | 0.33 | 0.7438 |
| WMH volume (ml) | 6.8 (9.5) | 9.8 (12.6) | 0.61 | 0.5481 |
| MMSE | 28.1 (1.3) | 28.6 (1.4) | 0.77 | 0.4529 |
| MADRS, baseline | 26.8 (4.8) | 28.0 (5.0) | 0.56 | 0.5806 |
| MADRS, final | 3.0 (3.2) | 18.8 (5.1) | 8.37 | <0.0001 |
| Cortical CBF, room air | 33.1 (4.5) | 34.6 (7.1) | 0.56 | 0.5796 |
| Cortical CVR | N=10 | N=8 | 0.84 | 0.4106 |
| 0.08 (0.09) | 0.11 (0.09) |
Continuous variables presented in mean (standard deviation), categorical as percent (number). Comparison of continuous variables made using pooled, two-tailed t-tests with 19 degrees of freedom, except for group comparisons of cortical CVR which had 16 degrees of freedom due to a smaller sample. Comparison of categorical variables (sex) made using a Fisher’s exact test. CBF = cerebral blood flow, CIRS = Cumulative Illness Rating Scale, CVR = cerebrovascular reactivity, MADRS = Montgomery-Asberg Depression Rating Scale, MMSE = Mini-Mental State Exam, WMH = white matter hyperintensity
3.1 Analyses of CBF
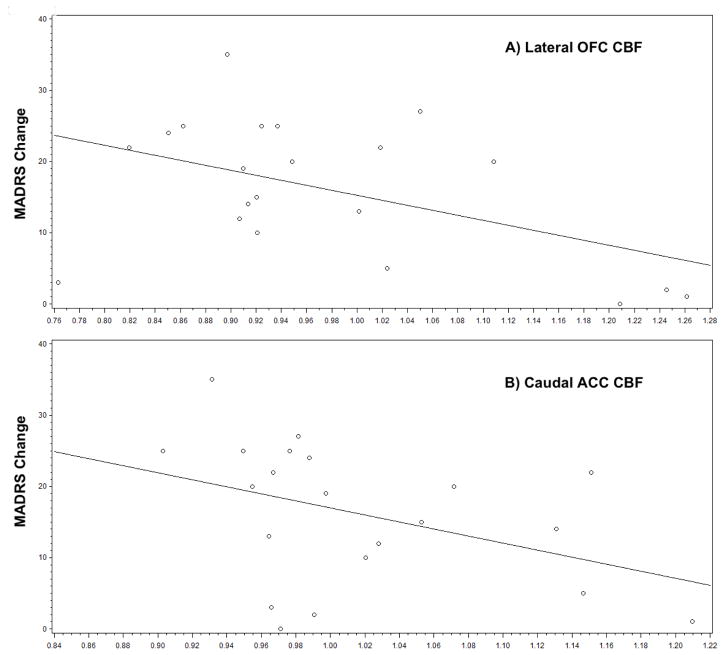
We first tested for a relationship between CBF and baseline MADRS. After controlling for age, baseline MADRS was not significantly associated with regional CBF in any frontal or cingulate region (data not shown). In subsequent analyses controlling for age and baseline MADRS, change in MADRS score was associated with CBF in the lateral orbitofrontal cortex (OFC) (F1,17 = 6.20, p = 0.0234) and caudal anterior cingulate cortex (cACC) (F1,17 = 7.19, p = 0.0157). In these models, regional CBF was negatively associated with subsequent MADRS change, so a higher baseline CBF was associated with less MADRS change (Fig. 2). Finally, we tested for differences in baseline CBF between remitters and nonremitters while controlling for age and baseline depression severity. We detected a statistically significant group difference in the cACC (Remitters (R): 0.98 (0.07); Nonremitters (NR): 1.05 (0.08); F1,17 = 4.59, p = 0.0469), but not in the lateral OFC (R: 0.93 (0.08); NR: 1.02 (0.17); F1,17 = 2.04 p = 0.1709) or other frontocingulate regions. Similar results were observed when examining response, defined as a 50% improvement in MADRS score, with a group difference observed in the cACC (Responders (N=12): 0.99 (0.07); Nonresponders (N=9): 1.06 (0.09); F1,17 = 4.62 p = 0.0463) but not lateral OFC (Responders (N=12): 0.94 (0.09); Nonresponders (N=9): 1.02 (0.18); F1,17 = 1.41, p = 0.2506).
Fig. 2.
Relationship between cerebral blood flow on room air and change in depression severity, Figures show relationship between regional cerebral blood flow (x-axis) and change in depression severity by MADRS (calculated as baseline MADRS – final MADRS). Both figures demonstrate that individuals with the highest CBF in the lateral OFC and caudal ACC exhibited the least change in depression severity with sertraline treatment.
3.2 Analyses of CVR
Hypercapnia data were available for 18 participants (10 remitting and 8 nonremitting participants). All participants exhibited the expected increase in exhaled CO2 levels during the hypercapnia challenge. We observed a mean exhaled CO2 level (EtCO2) on medical air of 43.06 mmHg (5.31) and a mean hypercapnic normoxia EtCO2 level of 49.33 mmHg (4.35), resulting in a mean increase of 6.28 mmHg (SD=2.30; t = 11.60, 17df, p < 0.0001). There were no significant differences in these measures between remitters and non-remitters.
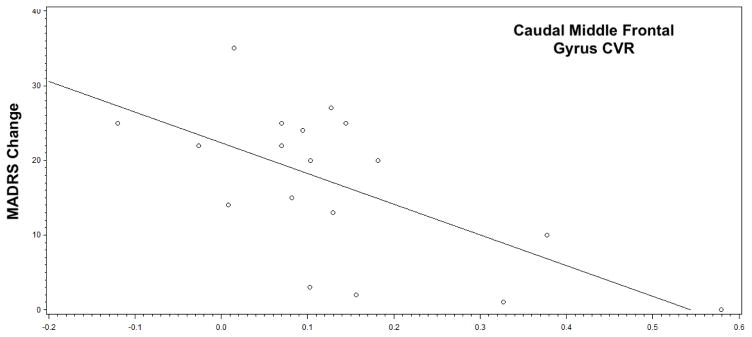
After controlling for age, we did not find any significant relationships between baseline MADRS and CVR in any frontocingulate region. Subsequent analyses controlling for age and baseline MADRS only found a statistically significant relationship between change in MADRS and caudal medial frontal gyrus (cMFG) CVR (F1,14 = 7.89, p = 0.0139). This was a negative relationship; greater CVR in the cMFG was associated with less MADRS change (Fig. 3). We also found that cMFG CVR differed by remission status (R: 0.068 (0.091); NR: 0.217 (0.193); F1,14 = 5.29, p = 0.0373) and response status, using a definition of 50% improvement in MADRS score (Responders (N=11): 0.072 (0.087); Nonresponders (N=7): 0.233 (0.203); F1,14 = 4.94, p = 0.0432). CVR did not significantly differ between remitting and nonremitting subjects in any other frontocingulate region.
Fig. 3.
Relationship between cerebrovascular reactivity and change in depression severity, Figure shows the relationship between caudal middle frontal gyrus CVR (calculated as the difference in CBF between hypercapnic normoxia and medical air conditions, divided by CBF on medical air) and change in depression severity by MADRS (calculated as baseline MADRS – final MADRS). Individuals with the highest CVR exhibited the least change in depression severity with sertraline treatment.
3.3. Effect of MRI hyperintensity volumes on observed relationships with CBF and CVR
In post-hoc exploratory analyses we first examined the relationship between WMH and ASL measures, focusing on the regions identified in the analyses described above. In models controlling for age, we found that WMH volume was not significantly associated with CBF of the lateral OFC or cACC (data not shown). In a similar model, we did not observe a relationship between WMH volume and cMFG CVR (data not shown).
Next we examined whether addition of WMH to models predicting change in MADRS over the study altered the observed relationships with ASL measures. Adding WMH volume to models described above did not significantly alter the observed relationship between change in depression severity and lateral OFC CBF, cACC CBF, or cMFG CVR (data not shown).
4. Discussion
In this pharmacoimaging ASL-MRI study, our primary finding was that higher pre-treatment CBF in the lateral OFC and cACC was associated with less change in depression severity over 12-weeks of open-label sertraline. Additionally, greater CVR (a greater change in CBF during hypercapnia) in the cMFG was also associated with less change in depression severity. Similar findings were observed when testing for differences between remitters and nonremitters. There were no frontocingulate regions where poor antidepressant response was related to lower CBF or lower CVR.
These findings are contrary to our initial hypothesis, as we anticipated that regional reductions in CBF and lower CVR would be associated with poor antidepressant response. These findings thus do not appear to support our Hypoperfusion Hypothesis (Taylor et al. 2013) that posits that reductions in CBF would be associated with the persistence of depressive symptoms. However, our association of increased CBF with poor antidepressant response is supported by studies associating higher perfusion in frontocingulate regions with either diagnoses of depression or poor response to treatment, including temporal structures such as the amygdala and hippocampus and frontal areas such as the inferior frontal gyrus and OFC (Chen et al. 2011; Duhameau et al. 2010; Weiduschat et al. 2013). The ACC has been similarly implicated in depression, with increased perfusion and metabolic activity identified in the subgenual ACC (Duhameau et al. 2010, Su et al. 2014) or subcallosal ACC (Ho et al. 2013) although these regions are not the same as observed in our study. In contrast to our hypothesis that reduced CBF would be related to vascular pathology, our findings of regionally increased CBF may represent underlying increases in metabolic activity of these regions (de la Torre 2012; Iadecola 2004).
Detection of a signal associating response with CBF in the OFC and cACC may be related to functional interactions between these regions. The OFC, which has been previously associated with LLD (Taylor et al. 2007), is involved in stimulus evaluation and encoding, playing a key role in the hedonic experience by representing the affective value of stimuli and contributing to decision making (Kringelbach 2005). It is functionally connected to the ACC, which is in turn involved with multiple cognitive processes including the encoding the subjective value of stimuli and action selection (Gleichgerrcht et al. 2010). Importantly, the OFC and ACC are structurally connected through the uncinate fasciculus, a fiber tract wherein damage is also associated with poor antidepressant response in older adults (Taylor et al. 2014b). Thus, these regions play a key role in decision making, and through further connections, are involved in the reward system. Imaging studies support alterations in the reward system in depression (Tremblay et al. 2005), while dysfunction of these regions is thought to impair executive function and decision making in late-life depression and neurodegenerative diseases (Gleichgerrcht et al. 2010; Elderkin-Thompson et al. 2009). Thus, while contrary to our initial hypothesis, these findings support that increased CBF in these regions, potentially as a marker of increased metabolic activity, may serve as a marker of a decreased likelihood of responding to a serotonin reuptake inhibitor. Supportingly, past PET studies have associated pre-treatment hyperactivity in the subgenual and pregenual ACC with failure to respond to antidepressant treatments (Konarski et al. 2009, Mayberg et al. 2005, Yang et al. 2009).
The functional significance of increased CVR in the caudal middle frontal gyrus (cMFG) being associated with poor antidepressant response is unclear. Again, this finding is contrary to our initial hypothesis and does not support that vascular pathology is contributing to dysfunction in this region. The cMFG is part of the broader dorsolateral prefrontal cortex, a region involved in attention, executive function, and cognitive control that is implicated in both functional connectivity and structural studies of LLD (Chang et al. 2011; Alexopoulos et al. 2012). Hypothetically, it is possible that increased CVR may reflect compensatory processes in a suboptimally performing region. However, this needs to be further examined in other studies, perhaps including other markers of vascular pathology.
As we focus on LLD, it is important to consider how these findings fit in context of aging. Normal aging is associated with increased perfusion of the anterior cingulate cortex and medial temporal lobe, but decreased perfusion in superior temporal regions and the OFC (Lee et al. 2009). Regional increases in CBF are hypothesized to be a compensatory response, potentially playing a role in early neuropathology. For example, although Mild Cognitive Impairment and Alzheimer’s Disease are generally associated with reduced parietal and temporal CBF (Zhang et al. 2017), some have observed regional hyperperfusion in asymptomatic individuals at risk for Alzheimer’s Disease (Ostergaard et al. 2013). Although an admittedly controversial hypothesis, some have proposed that increased CBF, not decreased CBF, may represent vascular pathology early in disease processes (Jespersen and Ostergaard 2011). In this model, microvascular disease may result in red blood cells traversing capillaries in a heterogeneous fashion with a higher overall velocity. This would result in red blood cells being present in the capillary bed for a shorter period, with oxygen transfer being less efficient, thus requiring a higher CBF to maintain required oxygen delivery. It is also possible that CVR may be higher because of similar processes.
To our knowledge, this is the first study to examine regional baseline measures of CBF and CVR as predictors of subsequent antidepressant response. Other studies have focused on general hemodynamic changes in relation to treatment. Some show CBF to be elevated in depression in temporal, frontal and anterior cingulate regions, and this may decrease after 6 weeks of escitalopram treatment (Kaichi et al. 2016). CVR is reportedly reduced in depression (Neu et al. 2004, Lemke et al. 2010) but tends to improve in those who remit to a euthymic state (Lemke et al. 2010).
Strengths of the study include use of ASL as an imaging modality and our standardized method of creating the hypercapnia challenge, resulting in the expected increase in exhaled carbon dioxide and allowing us to measure CVR. ASL MRI is low-risk, reliable and easy to use compared with other radiologic techniques that measure perfusion.
Our study also has limitations including a relatively small sample size that limits the power of our results and may have prevented us from detecting subthreshold findings, especially since LLD is a heterogenous diagnostic category. This limitation is somewhat mitigated as the remitted and nonremitted groups did not exhibit significant differences in clinical or medical variables. Moreover, as the majority of participants exhibited a mild to moderate level of depression severity, enrollment of a more severely affected cohort may have changed our conclusions. While we chose to focus on certain regions of interest (ROI) based on our background knowledge regarding the role they play in depression, we may have missed signals in other regions by failing to examine signals outside these ROIs. Additionally, as we this is an open-label study that did not include a placebo group, the relationship between ASL measures and clinical response is not necessarily entirely related to the drug effect. Placebo responses are common in depression trials, but occur through neural pathways that may be distinct from those involved in drug responses (Mayberg et al. 2002). Finally, in our effort to be comprehensive in our search for the various implicated regions, we did not adjust for multiple comparisons given the preliminary nature of these data. When considering the overall study in context of these limitations, study results should be viewed cautiously. These findings require replication using a more rigorous controlled trial design.
5. Conclusions
Our study in a LLD population does not support that cerebral hypoperfusion is associated with response to antidepressants. However, we did find regions where increased baseline CBF was associated with poor antidepressant response. Prior work associates increased metabolism in these regions with poor response. While our CBF findings may reflect differences in metabolic activity in these areas, this is another hypothesis that warrants further corroboration. Future work should incorporate additional measures to obtain a broader view of CBF in context of bran metabolism. It should also examine whether the hypoperfusion hypothesis may inform us of vulnerability to depression or whether it is related to the cognitive presentation of LLD.
Highlights.
Vascular pathology is greater in and mechanistically related to late-life depression.
Cerebrovascular reactivity (CVR) is a measure of dynamic vascular processes.
Frontocingulate cerebral blood flow (CBF) is associated with antidepressant response.
Greater CBF may reflect greater regional metabolic activity.
Acknowledgments
Funding Support: This research was supported by NIH grants R21 MH099218 and K24 MH110598, and by CTSA award UL1TR000445 from the National Center for Advancing Translational Sciences.
Footnotes
No disclosures to report.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alexopoulos G, Meyers B, Young R, Campbell S, Silbersweig D, Charlson M. ‘Vascular depression’ hypothesis. Arch Gen Psychiatry. 1997;54:915–922. doi: 10.1001/archpsyc.1997.01830220033006. [DOI] [PubMed] [Google Scholar]
- Alexopoulos GS, Hoptman MJ, Kanellopoulos D, Murphy CF, Lim KO, Gunning FM. Functional connectivity of the cognitive control network and the default mode network in late-life depression. J Affect Disord. 2012;139:56–65. doi: 10.1016/j.jad.2011.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alsop DC, Detre JA, Golay X, Gunther M, Hendrikse J, Hernandez-Garcia L, Lu H, MacIntosh BJ, Parkes LM, Smits M, van Osch MJP, Wang DJJ, Wong EC, Zaharchuk G. Recommended implementation of arterial spin labeled perfusion MRI for clinical applications: a consensus of the ISMRM perfusion study group and the European consortium for ASL in dementia. Magnet Reson Med. 2015;73:102–116. doi: 10.1002/mrm.25197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brockmann H, Zobel A, Joe A, Biermann K, Scheef L, Schuhmacher A, von Widdern O, Metten M, Biersack HJ, Maier W, Boecker H. The value of HMPAO SPECT in predicting treatment response to citalopram in patients with major depression. Psychiatry Res. 2009;173(2):107–12. doi: 10.1016/j.pscychresns.2008.10.006. [DOI] [PubMed] [Google Scholar]
- Chang CC, Yu SC, McQuoid DR, Messer DF, Taylor WD, Singh K, Boyd BD, Krishnan KRR, MacFall JR, Steffens DC, Payne ME. Reduction of dorsolateral prefrontal cortex gray matter in late-life depression. Psychiatry Res Neuroimag. 2011;193:1–6. doi: 10.1016/j.pscychresns.2011.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Wan HI, O’Reardon JP, Wang DJJ, Wang Z, Korczykowski M, Detre JA. Quantification of cerebral blood flow as biomarker of drug effect: arterial spin labeling phMRI after a single dose of oral citalopram. Clin Pharmacol Ther. 2011;89:251–258. doi: 10.1038/clpt.2010.296. [DOI] [PubMed] [Google Scholar]
- Chi KF, Korgaonkar M, Grieve SM. Imaging predictors of remission to anti-depressant medications in major depressive disorder. J Affect Disord. 2015;186:134–144. doi: 10.1016/j.jad.2015.07.002. [DOI] [PubMed] [Google Scholar]
- Colloby SJ, Firbank MJ, He J, Thomas AJ, Vasudev A, Parry SW, O’Brien JT. Regional cerebral blood flow in late-life depression: arterial spin labeling magnetic resonance study. Br J Psychiatry. 2012;200:150–155. doi: 10.1192/bjp.bp.111.092387. [DOI] [PubMed] [Google Scholar]
- Dandona P, Chaudhuri A, Aljada A. Endothelial dysfunction and hypertension in diabetes mellitus. Med Clin North Am. 2004;88:911–931. doi: 10.1016/j.mcna.2004.04.006. [DOI] [PubMed] [Google Scholar]
- de la Torre JC. Cerebral hemodynamics and vascular risk factors: setting the stage for Alzheimer’s disease. J Alzheimers Dis. 2012;32:553–567. doi: 10.3233/JAD-2012-120793. [DOI] [PubMed] [Google Scholar]
- Debiec J, LeDoux JE, Nader K. Cellular and systems reconsolidation in the hippocampus. Neuron. 2002;36:527–538. doi: 10.1016/s0896-6273(02)01001-2. [DOI] [PubMed] [Google Scholar]
- Direk N, Koudstaal PJ, Hofman A, Ikram MA, Hoogendijk WJ, Tiemeier H. Cerebral hemodynamics and incident depression: the rotterdam study. Biol Psychiatry. 2012;72:318–323. doi: 10.1016/j.biopsych.2012.01.019. [DOI] [PubMed] [Google Scholar]
- Donahue MJ, Faraco CC, Strother MK, Chappell MA, Rane S, Dethrage LM, Hendrikse J, Siero JCW. Bolus arrival time and cerebral blood flow responses to hypercarbia. J Cereb Blood Flow Metab. 2014;34:1243–1252. doi: 10.1038/jcbfm.2014.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duhameau B, Ferré JC, Jannin P, Gauvrit JY, Vérin M, Millet B, Drapier D. Chronic and treatment-resistant depression: A study using arterial spin labeling perfusion MRI at 3Tesla. Psychiatry Res Neuroimag. 2010;182:111–116. doi: 10.1016/j.pscychresns.2010.01.009. [DOI] [PubMed] [Google Scholar]
- Elderkin-Thompson V, Hellemann G, Pham D, Kumar A. Prefrontal brain morphology and executive function in healthy and depressed elderly. Int J Geriatr Psychiatry. 2009;24:459–468. doi: 10.1002/gps.2137. [DOI] [PubMed] [Google Scholar]
- Fischl B. Automatically parcellating the human cerebral cortex. Cereb Cortex. 2004;14:11–22. doi: 10.1093/cercor/bhg087. [DOI] [PubMed] [Google Scholar]
- Fischl B, Salat DH, van der Kouwe AJW, Makris N, Ségonne F, Quinn BT, Dale AM. Sequence-independent segmentation of magnetic resonance images. Neuroimage. 2004;23:S69–S84. doi: 10.1016/j.neuroimage.2004.07.016. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. Mini-mental state. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Gleichgerrcht E, Ibáñez A, Roca M, Torralva T, Manes F. Decision-making cognition in neurodegenerative diseases. Nat Rev Neurol. 2010;6:611–623. doi: 10.1038/nrneurol.2010.148. [DOI] [PubMed] [Google Scholar]
- Glodzik L, Rusinek H, Brys M, Tsui WH, Switalski R, Mosconi L, Mistur R, Pirraglia E, de Santi S, Li Y, et al. Framingham cardiovascular risk profile correlates with impaired hippocampal and cortical vasoreactivity to hypercapnia. J Cereb Blood Flow Metab. 2011;31:671–679. doi: 10.1038/jcbfm.2010.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenstein AS, Paranthaman R, Burns A, Jackson A, Malik RA, Baldwin RC, Heagerty AM. Cerebrovascular damage in late-life depression is associated with structural and functional abnormalities of subcutaneous small arteries. Hypertension. 2010;56:734–740. doi: 10.1161/HYPERTENSIONAHA.110.152801. [DOI] [PubMed] [Google Scholar]
- Hajjar I, Zhao P, Alsop D, Novak V. Hypertension and cerebral vasoreactivity: A continuous arterial spin labeling magnetic resonance imaging study. Hypertension. 2010;56:859–864. doi: 10.1161/HYPERTENSIONAHA.110.160002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heo S, Prakash RS, Voss MW, Erickson KI, Ouyang C, Sutton BP, Kramer AF. Resting hippocampal blood flow, spatial memory and aging. Brain Res. 2010;1315:119–127. doi: 10.1016/j.brainres.2009.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho TC, Wu J, Shin DD, Liu TT, Tapert SF, Yang G, Connolly CG, Frank GK, Max JE, Wolkowitz O, Eisendrath S, Hoeft F, Banerjee D, Hood K, Hendren RL, Paulus MP, Simmons AN, Yang TT. Altered cerebral perfusion in executive, affective, and motor networks during adolescent depression. J Am Acad Child Adolesc Psychiatry. 2013;52:1076–1091. doi: 10.1016/j.jaac.2013.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iadecola C. Neurovascular regulation in the normal brain and in Alzheimer’s disease. Nat Rev Neurosc. 2004;5:347–360. doi: 10.1038/nrn1387. [DOI] [PubMed] [Google Scholar]
- Jespersen SN, Ostergaard L. The roles of cerebral blood flow, capillary transit time heterogeneity, and oxygen tension in brain oxygenation and metabolism. J Cereb Blood Flow Metab. 2012;32:264–277. doi: 10.1038/jcbfm.2011.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaichi Y, Okada G, Takamura M, Toki S, Akiyama Y, Higaki T, Matsubara Y, Okamoto Y, Yamawaki S, Awai K. Changes in the regional cerebral blood flow detected by arterial spin labeling after 6-week escitalopram treatment for major depressive disorder. J Affect Disord. 2016;194:135–143. doi: 10.1016/j.jad.2015.12.062. [DOI] [PubMed] [Google Scholar]
- Kleim JA, Bruneau R, Calder K, Pocock D, VandenBerg PM, MacDonald E, Monfils MH, Sutherland RJ, Nader K. Functional organization of adult motor cortex is dependent upon continued protein synthesis. Neuron. 2003;40:167–176. doi: 10.1016/s0896-6273(03)00592-0. [DOI] [PubMed] [Google Scholar]
- Konarski JZ, Kennedy SH, Segal ZV, Lau MA, Bieling PJ, McIntyre RS, Mayberg HS. Differences in brain glucose metabolism between responders to CBT and venlafaxine in a 16-week randomized controlled trial. Am J Psychiatry. 2007;164:778–788. doi: 10.1176/ajp.2007.164.5.778. [DOI] [PubMed] [Google Scholar]
- Kringelbach ML. The human orbitofrontal cortex: linking reward to hedonic experience. Nat Rev Neurosc. 2005;6:691–702. doi: 10.1038/nrn1747. [DOI] [PubMed] [Google Scholar]
- Lee C, Lopez OL, Becker JT, Raji C, Dai W, Kuller LH, Gach HM. Imaging cerebral blood flow in the cognitively normal aging brain with arterial spin labeling: implications for imaging of neurodegenerative disease. J Neuroimaging. 2009;19(4):344–52. doi: 10.1111/j.1552-6569.2008.00277.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemke H, de Castro AG, Schlattmann P, Heuser I, Neu P. Cerebrovascular reactivity over time-course – from major depressive episode to remission. J Psychiatr Res. 2010;44(3):132–136. doi: 10.1016/j.jpsychires.2009.06.010. [DOI] [PubMed] [Google Scholar]
- Lu H, Xu F, Rodrigue KM, Kennedy KM, Cheng Y, Flicker B, Hebrank AC, Uh J, Park DC. Alterations in cerebral metabolic rate and blood supply across the adult lifespan. Cereb Cortex. 2011;21:1426–1434. doi: 10.1093/cercor/bhq224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lui S, Parkes LM, Huang X, Zou K, Chan RC, Yang H, Zou L, Li D, Tang H, Zhang T, et al. Depressive disorders: focally altered cerebral perfusion measured with arterial spin-labeling MR imaging. Radiology. 2009;251:476–484. doi: 10.1148/radiol.2512081548. [DOI] [PubMed] [Google Scholar]
- Mayberg HS, Silva JA, Brannan SK, Tekell JL, Mahurin RK, McGinnis S, Jerabek PA. The functional neuroanatomy of the placebo effect. Am J Psychiatry. 2002;159:728–737. doi: 10.1176/appi.ajp.159.5.728. [DOI] [PubMed] [Google Scholar]
- Mayberg HS, Lozano AM, Voon V, McNeely HE, Seminowicz D, Hamani C, Schwalb JM, Kennedy SH. Deep brain stimulation for treatment-resistant depression. Neuron. 2005;45:651–660. doi: 10.1016/j.neuron.2005.02.014. [DOI] [PubMed] [Google Scholar]
- Miller MD, Paradis CF, Houck PR, Mazumdar S, Stack JA, Rifai AH, Mulsant B, Reynolds CF. Rating chronic medical illness burden in geropsychiatric practice and research: application of the cumulative illness rating scale. Psychiatry Res. 1992;41:237–248. doi: 10.1016/0165-1781(92)90005-n. [DOI] [PubMed] [Google Scholar]
- Monkul ES, Silva LAP, Narayana S, Peluso MAM, Zamarripa F, Nery FG, Najt P, Li J, Lancaster JL, Fox PT, Lafer B, Soares JC. Abnormal resting state corticolimbic blood flow in depressed unmedicated patients with major depression: A 15O-H2O PET study. Hum Brain Mapp. 2012;33:272–279. doi: 10.1002/hbm.21212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montgomery S, Asberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry. 1979;134:382–389. doi: 10.1192/bjp.134.4.382. [DOI] [PubMed] [Google Scholar]
- Neu P, Schlattmann P, Schilling A, Hartmann A. Cerebrovascular reactivity in major depression – a pilot-study. Psychosomatic med. 2004;66:6–8. doi: 10.1097/01.psy.0000107880.03026.54. [DOI] [PubMed] [Google Scholar]
- Noguchi T, Kawashima M, Irie H, Ootsuka T, Nishihara M, Matsushima T, Kudo S. Arterial spin-labeling MR imaging in moyamoya disease compared with SPECT imaging. Eur J Radiol. 2011;80:e557–e562. doi: 10.1016/j.ejrad.2011.01.016. [DOI] [PubMed] [Google Scholar]
- Ota M, Noda T, Sato N, Hattori K, Teraishi T, Hori H, Nagashima A, Shimoji K, Higuchi T, Kunugi H. Characteristic distributions of regional cerebral blood flow changes in major depressive disorder patients: a pseudo-continuous arterial spin labeling (pCASL) study. J Affect Disord. 2014;165:59–63. doi: 10.1016/j.jad.2014.04.032. [DOI] [PubMed] [Google Scholar]
- Ostergaard L, Aamand R, Gutierrez-Jimenez E, Ho YL, Blicher JU, Madsen SM, Nagenthiraja K, Dalby RB, Drasbek KR, Moller A, Braendgaard H, Mouridsen K, Jespersen SN, Jensen MS, West MJ. The capillary dysfunction hypothesis of Alzheimer’s disease. Neurobiol Aging. 2013;34:1018–1031. doi: 10.1016/j.neurobiolaging.2012.09.011. [DOI] [PubMed] [Google Scholar]
- Paranthaman R, Greenstein AS, Burns AS, Cruickshank JK, Heagerty AM, Jackson A, Malik RA, Scott MLJ, Baldwin RC. Vascular function in older adults with depressive disorder. Biol Psychiatry. 2010;68:133–139. doi: 10.1016/j.biopsych.2010.04.017. [DOI] [PubMed] [Google Scholar]
- Park JH, Lee SB, Lee JJ, Yoon JC, Han JW, Kim TH, Jeong HG, Newhouse PA, Taylor WD, Kim JH, Woo JI, Kim KW. Epidemiology of MRI-defined vascular depression: A longitudinal, community-based study in Korean elders. J Affect Disord. 2015;180:200–206. doi: 10.1016/j.jad.2015.04.008. [DOI] [PubMed] [Google Scholar]
- Périco CA, Skaf CR, Yamada A, Duran F, Buchpiguel CA, Castro CC, Soares JC, Busatto GF. Relationship between regional cerebral blood flow and separate symptom clusters of major depression: a single photon emission computed tomography study using statistical parametric mapping. Neurosci Lett. 2005;384(3):265–70. doi: 10.1016/j.neulet.2005.04.088. [DOI] [PubMed] [Google Scholar]
- Rabbitt P, Scott M, Thacker N, Lowe C, Jackson A, Horan M, Pendleton N. Losses in gross brain volume and cerebral blood flow account for age-related differences in speed but not in fluid intelligence. Neuropsychology. 2006;20:549–557. doi: 10.1037/0894-4105.20.5.549. [DOI] [PubMed] [Google Scholar]
- Schmidt P, Gaser C, Arsic M, Buck D, Forschler A, Berthele A, Hosi M, Ilg R, Schmid VJ, Zimmer C, Hemmer B, Muhlau M. An automated tool for detection of FLAIR-hyperintense white-matter lesions in Multiple Sclerosis. Neuroimage. 2012;59:3774–3783. doi: 10.1016/j.neuroimage.2011.11.032. [DOI] [PubMed] [Google Scholar]
- Sheehan D, Lecrubier Y, Sheehan H, Amorim P, Janavs J, Weiller E, Hergueta T, Baker R, Dunbar G. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnotic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59:22–23. [PubMed] [Google Scholar]
- Silvestrini M, Pasqualetti P, Baruffaldi R, Bartolini M, Handouk Y, Matteis M, Moffa F, Provinciali L, Vernieri F. Cerebrovascular reactivity and cognitive decline in patients with alzheimer disease. Stroke. 2006;37:1010–1015. doi: 10.1161/01.STR.0000206439.62025.97. [DOI] [PubMed] [Google Scholar]
- Su L, Cai Y, Xu Y, Dutt A, Shenxun S, Bramon E. Cerebral metabolism in major depressive disorder: a voxel-based meta-analysis of positron emission tomography studies. BMC Psychiatry. 2014;14:321–328. doi: 10.1186/s12888-014-0321-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor WD, McQuoid DR, Krishnan KRR. Medical comorbidity in late-life depression. Int J Geriatr Psychiatry. 2004;19:935–943. doi: 10.1002/gps.1186. [DOI] [PubMed] [Google Scholar]
- Taylor WD, MacFALL JR, Payne ME, McQUOID DR, Steffens DC, Provenzale JM, Krishnan KRR. Orbitofrontal cortex volume in late life depression: influence of hyperintense lesions and genetic polymorphisms. Psychol Med. 2007;37:1763–1773. doi: 10.1017/S0033291707000128. [DOI] [PubMed] [Google Scholar]
- Taylor WD, Aizenstein HJ, Alexopoulos GS. The vascular depression hypothesis: mechanisms linking vascular disease with depression. Mol Psychiatry. 2013;18:963–974. doi: 10.1038/mp.2013.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor WD. Depression in the Elderly. N Engl J Med. 2014a;371:1228–1236. doi: 10.1056/NEJMcp1402180. [DOI] [PubMed] [Google Scholar]
- Taylor WD, Kudra K, Zhao Z, Steffens DC, MacFall JR. Cingulum bundle white matter lesions influence antidepressant response in late-life depression: A pilot study. J Affect Disord. 2014b;162:8–11. doi: 10.1016/j.jad.2014.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiemeier H. Cerebral haemodynamics and depression in the elderly. J Neurol Neurosurg Psychiatry. 2002;73:34–39. doi: 10.1136/jnnp.73.1.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremblay LK, Naranjo CA, Graham SJ, Herrmann N, Mayberg HS, Hevenor S, Busto UE. Functional neuroanatomical substrates of altered reward processing in major depressive disorder revealed by a dopaminergic probe. Arch Gen Psychiatry. 2005;62:1228–1236. doi: 10.1001/archpsyc.62.11.1228. [DOI] [PubMed] [Google Scholar]
- Videbech P, Raynkilde B, Pedersen T, Hartvig H, Egander A, Clemmensen K, Rasmussen N, Andersen F, Gjedde A, Rosenberg R. The Danish PET/depression project: Clinical symptoms and cerebral blood flow. A regions-of-interest analysis. Acta Psychiatr Scand. 2002;106:35–39. doi: 10.1034/j.1600-0447.2002.02245.x. [DOI] [PubMed] [Google Scholar]
- Weiduschat N, Dubin MJ. Prefrontal cortical blood flow predicts response of depression to rTMS. J Affect Disord. 2013;150:699–702. doi: 10.1016/j.jad.2013.04.049. [DOI] [PubMed] [Google Scholar]
- Williams DS, Detre JA, Leigh JS, Koretsky AP. Magnetic resonance imaging of perfusion using spin inversion of arterial water. Proc Natl Acad Sci. 1992;89:212–216. doi: 10.1073/pnas.89.1.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf PA, D’Agostino RB, Belanger AJ, Kannel WB. Probability of stroke: a risk profile from the Framingham Study. Stroke. 1991;22:312–318. doi: 10.1161/01.str.22.3.312. [DOI] [PubMed] [Google Scholar]
- Yang TT, Simmons AN, Matthews SC, Tapert SF, Frank GK, Bischoff-Grethe A, Lansing AE, Wu J, Brown GG, Paulus MP. Depressed adolescents demonstrate greater subgenual anterior cingulate activity. Neuroreport. 2009;20:440–444. doi: 10.1097/WNR.0b013e3283262e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaharchuk G, Mandeville JB, Bogdanov AA, Weissleder R, Rosen BR, Marota JJA, Iadecola C, Kim SG. Cerebrovascular dynamics of autoregulation and hypoperfusion : An MRI study of CBF and changes in total and microvascular cerebral blood volume during hemorrhagic hypotension. Stroke. 1999;30:2197–2205. doi: 10.1161/01.str.30.10.2197. [DOI] [PubMed] [Google Scholar]
- Zhang N, Gordon ML, Goldberg TE. Cerebral blood flow measured by arterial spin labeling MRI at resting state in normal aging and Alzheimer’s disease. Neurosci Biobehav Rev. 2017;72:168–175. doi: 10.1016/j.neubiorev.2016.11.023. [DOI] [PubMed] [Google Scholar]