Abstract
Objective:
Cancer patients often experience a large number of symptoms together. The aim of this study is to determine the symptom clusters in cancer patients at palliative care clinic.
Methods:
Hundred and seventy consecutive patients were enrolled in the study. Memorial Symptom Assessment Scale was used for symptom assessment of the patients.
Results:
The most experienced symptoms by the patients during the past week before hospitalization in palliative care clinic were lack of energy (95.4%), weight loss (91.2%), lack of appetite (89.4%), pain (88.2%), dry mouth (87.6%), feeling sad (87.6%), feeling nervous (82.9%), worrying (81.2%), and feeling irritable (80.6%). Five symptom clusters were defined. First cluster: pain, feeling nervous, dry mouth, worrying, feeling irritable, weight loss; second cluster: feeling drowsy, numbness/tingling in hands/feet, difficulty in sleeping, dizziness, constipation, I do not look like myself; third cluster: nausea, vomiting; fourth cluster: shortness of breath, difficulty in swallowing, cough, change in the way food tastes; and fifth cluster: feeling bloated, problems with urination, diarrhea, itching, mouth sores, hair loss, swelling of arm or legs, change in the skin.
Conclusions:
We encountered various symptom clusters in advanced cancer patients. Identification of symptom clusters and knowledge of cluster composition in oncological population may particularly contribute individualization of the treatment.
Keywords: Cluster, palliative care, symptom

Introduction
Cancer patients often experience a large number of symptoms together which may be difficult to manage from the diagnosis to the treatment or terminal phase of the illness. Patients with cancer rarely present with a single symptom affecting the quality of life.[1,2] The symptoms coexisting in advanced cancer patients usually produce symptom groups by interacting with each other. This interrelation among the symptoms led to symptom clustering which has become a current issue in the last years. The term symptom cluster has been defined as coexistence of two or more related symptoms which may or may not suggest a common etiology or underlying mechanism.[1] It has been reported that understanding the association among the symptom clusters would result in better symptom control and improvement in the quality of life and treating symptoms individually. By understanding symptom clusters, oncology nurses can develop more comprehensive assessments and set priorities in treatment planning, lessen the total symptom burden.[3,4,5]
One of the main objectives of palliative care is to provide optimal symptom relief and improve the life quality of patients. Palliative care clinics mainly hospitalize patients with more intense symptoms which are quite difficult to manage at home or as outpatient. Knowledge of symptom clusters is important as it may help the medical team to identify the most prevalent symptoms and anticipate appropriate care plan. More easy and effective treatment for symptom management can be achieved by expanding the treatment and care plan to cover the entire symptoms. The aim of this study is to determine the symptom clusters in cancer patients hospitalized at palliative care clinic.
Methods
Sample and design
This descriptive study was conducted in Turkey with a sample of hundred and seventy inpatients at palliative care clinic. Participants who were aged 18 years old or above, conscious, and gave consent for the study were prospectively included. The study was approved by the Institutional Review Board of Dr. AY Ankara Oncology Education and Research Hospital. Data were collected through a questionnaire that includes the defining participant characteristics and memorial symptom assessment scale (MSAS).
Descriptive data form
Demographics and disease-related characteristics (age, gender, diagnosis, time of diagnosis, presence of metastasis, previous treatment, time of diagnosis, time from the last antineoplastic treatment, chronic diseases) were recorded.
Memorial symptom assessment scale
MSAS was applied to the patients on the day of admission to palliative care clinic. MSAS is a multidimensional scale with 32 items and has Turkish reliability and validity study.[6] It is used for symptom assessment of cancer patients during the last week. Twenty-four symptoms are evaluated with respect to frequency, intensity, and distress, and eight symptoms are evaluated in terms of severity and distress. MSAS subscales include the Global Distress Index (GDI), physical symptom distress scores (PHYS), and psychological symptom distress scores (PSYCH). The GDI is the average of the frequency of four psychological symptoms (feeling sad, worrying, feeling irritable, and feeling nervous) and the distress associated with six physical symptoms (lack of appetite, lack of energy, pain, feeling drowsy, constipation, and dry mouth). The PHYS is the average of the score for the 12 symptoms: lack of appetite, lack of energy, pain, feeling drowsy, constipation, dry mouth, nausea, vomiting, change in taste, weight loss, feeling bloated, and dizziness. The PSYCH is the average of the score for the six symptoms: worrying, feeling sad, feeling nervous, difficulty in sleeping, feeling irritable, and difficulty in concentrating.
Statistical analysis
Statistical analysis was performed with IBM SPSS for Windows Version 21.0 program. Quantitative variables are expressed as mean ± standard deviation and median value (minimum-maximum). Categorical variables were shown by numbers and percentages. The average value was used for score distribution of Palliative Performance Scale and MSAS. The Kolmogorov–Smirnov normality test was applied to the scores calculated to determine the analyses to be performed. The test results showed that the scores provided normality assumption (P > 0.05), and for this reason, parametric tests were used in the comparison. Hierarchical cluster analysis was used to create the symptom clusters. The symptoms with <20% prevalence were excluded from the cluster analysis. The distance between the clusters was measured with Euclidean distance. The created clusters were shown by dendrogram.
Results
Patient characteristics
The mean age of the patients included in the study was 61 ± 13 years; 56% of them were male and 44% were female. The most common cancer was gastrointestinal tract tumors (26%) followed by lung (20.2%), genitourinary tract (17.6%), and head and neck cancer (11.2%). It was found that 52% of patients were diagnosed over a year ago, and 90% of the patients were metastatic. 40.6% of patients had chronic disease other than cancer. It was found that 23% of patients were not given antineoplastic treatment due to advanced stage and loss of performance scale. The time elapsed from the last antineoplastic treatment to hospitalization in palliative care clinic was <3 months in 32.6% of patients, 3–6 months in 25.9%, 7–12 months in 17.8%, and >12 months in 23.7%. Patients' average palliative performance scale score was 40 [Table 1].
Table 1.
Frequency of demographic characteristics
| Characteristics | n=170 (%) |
|---|---|
| Age (Mean±SD, years) | 61±13 |
| Gender | |
| Male | 95 (56.0) |
| Female | 75 (44.0) |
| Primary cancer site | |
| Gastrointestinal tract | 44 (26.0) |
| Lung | 36 (20.2) |
| Genitourinary tract | 30 (17.6) |
| Head and neck | 19 (11.2) |
| Breast | 16 (10.4) |
| Hepatobiliary | 16 (10.4) |
| Others | 9 (4.2) |
| Time of diagnosis (months) | |
| 0-3 | 28 (16.5) |
| 4-6 | 27 (15.9) |
| 7-12 | 26 (15.3) |
| ≥12 | 89 (52.4) |
| Metastasis | |
| Yes | 153 (90.0) |
| No | 17 (10.0) |
| Chronic illness | |
| Yes | 69 (41.6) |
| No | 101 (59.4) |
| The most recent treatment | |
| Chemotherapy | 40 (23.5) |
| Chemoradiotherapy | 55 (32.4) |
| Radiotherapy | 19 (11.2) |
| Surgery | 16 (9.4) |
| No treatment | 40 (23.5) |
| Completion period of treatment (months) | |
| 0-3 | 56 (32.6) |
| 4-6 | 44 (25.9) |
| 7-12 | 30 (17.8) |
| ≥12 | 40 (23.7) |
SD: Standard deviation
Symptom assessment
The most experienced symptoms by the patients during the past week before hospitalization in palliative clinic care were lack of energy (95.4%), weight loss (91.2%), lack of appetite (89.4%), pain (88.2%), dry mouth (87.6%), feeling sad (87.6%), feeling nervous (82.9%), worrying (81.2%), and feeling irritable (80.6%) The symptoms experienced almost constantly during the past week were found to be feeling sad (59.1%), lack of energy (56.2%), feeling irritable (49.6%), and lack of appetite (49.3%); rarely experienced symptoms were problems with sexual interest or activity (68.2%) and mouth sores (26.3%). With regard to the severity of the symptoms in the past week, feeling sad (61.1%) was the most expressed followed by feeling irritable (50.4%), lack of appetite (45.4%), and lack of energy (44.5%). The most distressing symptom experienced by the patients during the past week were feeling sad (57.7%), lack of energy (44.5%), I do not look like myself (48.5%), feeling irritable (48.2%), and lack of appetite (43.1%). Five symptom clusters were defined as the result of cluster analysis. The symptom clusters are shown by dendrogram in Figure 1. 1st cluster: pain, feeling nervous, dry mouth, worrying, feeling irritable, weight loss; 2nd cluster: feeling drowsy, numbness/tingling in hands/feet, difficulty in sleeping, dizziness, constipation, I do not look like myself; 3rd cluster: nausea, vomiting; 4th cluster: shortness of breath, difficulty in swallowing, cough, change in the way food tastes; and 5th cluster: feeling bloated, problems with urination, diarrhea, itching, mouth sores, hair loss, swelling of arm or legs, change in the skin.
Figure 1.
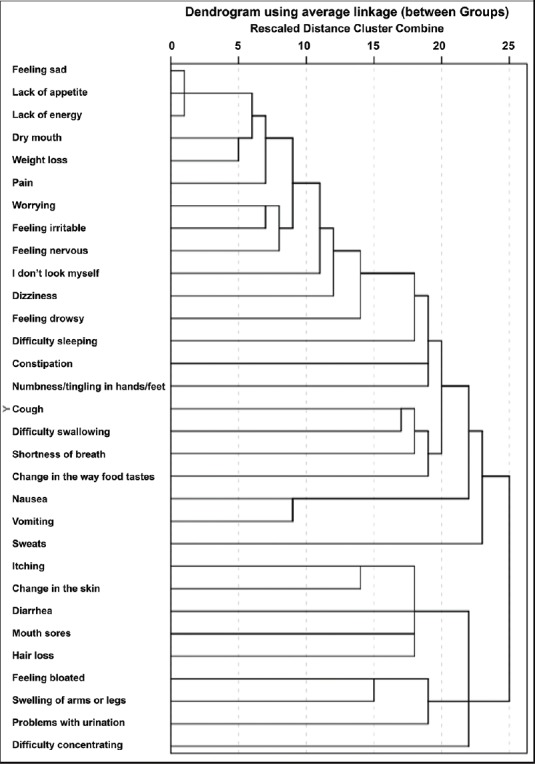
Symptom clusters of the patients during the last week
The mean scores of the subgroups of the Memorial Symptom Diagnosis Scale are shown in Table 2. There was a statistically significant difference between women and men according to the MSAS. Physical point averages (P < 0.05). According to this, we can say that women's MSTS physical point averages are significantly higher than men's point averages. There was no statistically significant difference between the mean scores of the subgroups of the Memorial Symptom Assessment Scale Memorial GDI, PHYS, and PSYCH between age, stage, chronic disease, and diagnostic time.
Table 2.
The mean scores of the subgroups of the Memorial Symptom Assessment Scale
| MSAS subgroups | Minimum–maximum | Mean±SD |
|---|---|---|
| GDI | 1.0-3.88 | 2.85±0.647 |
| PHYS | 1.0-3.75 | 2.64±0.609 |
| PSYCH | 1.0-4.00 | 3.05±0.664 |
| TMSAS | 0.87-3.82 | 2.64±0.638 |
TMSAS: Total Memorial Symptom Assessment Scale, PSYCH: Psychological symptom subscale score, PHYS: Physical symptom subscale score, GDI: Global distress index score, SD: Standard deviation
Discussion
In this study, it was found that 90% metastatic advanced stage cancer patients and their average palliative performance score was 40. Patients who did not receive antineoplastic treatment (23%) were thought to be associated with late cancer diagnosis. The high proportion of advanced cancers may also be due to poor outcomes of cancer diagnosis of participants. Furthermore, gastrointestinal, lung, head and neck, and hepatobiliary malignancies are usually associated with poor prognosis.
Cancer patients have been reported to experience an average of 11–13 concurrent symptoms.[7,8] Dodd and colleagues were among the first to use the term “symptom clusters” in their work with pain, fatigue, and sleep disturbances.[1] The presence of multiple coexisting symptoms may have an adverse effect on disease prognosis or treatment of one symptom may be affected by other components in the cluster.[4] We have assessed 32 symptoms in the present study. In previous studies, fewer symptoms were addressed to investigate symptom clustering in advanced cancer patients when compared to our study.[9,10] Symptoms related to sexual interest and activity were not included in the cluster analysis due to the low incidence of reporting (<20%). We thought that regarding the performance of patients, frequency, and severity of symptoms, patients were not able to express or evaluate their problems comfortably about sexual activity. Furthermore, ıt can be interpreted that the health workers neglect sexuality issue because Turkey is also a country where sexuality is taboo.
The first cluster consisted of the symptoms pain, feeling nervous, dry mouth, worrying, feeling irritable, and weight loss. It was observed that the incidence of symptoms in the first cluster was over 80%, and the frequency and severity of these symptoms were also high. The symptoms forming cluster with pain showed differences in this study. Previous studies have reported pain to cluster with lack of energy and constipation[11,12,13] as well as presenting as a single group without clustering.[14] Pain detraction is considered to be most important symptom impact on quality of life.[15] It is reported to be more severe during the terminal period with an incidence of 70%–90%.[16,17] The coexistence of pain with symptoms such as feeling nervous, worrying, and feeling irritable is not surprising. Dry mouth is thought to be associated with the drugs used for the treatment of pain and anxiety. However, the symptoms such as constipation and feeling drowsy which might be associated with opioid use were not in this group. Weight loss interestingly took place in the first cluster.
Although weight loss, lack of energy, and lack of appetite were the most common three symptoms, they did not form a cluster together. Lack of appetite is thought to be associated with overproduction of cytokines such as interferon, tumor necrosis factor, interleukin (IL-1), and IL-6.[18] It is usually the main cause of complaint for patient and his/her family and has an incidence of 40%–60% in the advanced disease.[19,20] In the present study, it was found as 89.4%. The symptoms such as lack of energy, loss of appetite, change in the way food taste, difficulty swallowing, weight loss, constipation, and dry mouth were reported to be coexisting symptoms associated with poor oral intake and lack of energy in previous studies classifying symptoms according to pathophysiology.[10,14] Lack of energy and lack of appetite did not participate in any cluster in our study. Similarly, difficulty in concentrating, feeling sad, and sweats were also excluded from the study.
Nausea and vomiting produced the third cluster separately, independent from other symptoms. In palliative care settings, many etiologies such as use of opioids, liver or kidney dysfunction, peritonitis, carcinomatosis, brain tumors, and gastrointestinal obstruction may cause nausea and vomiting. The incidence of nausea and vomiting is reported as 6%–37% in advanced stage cancer patients.[21,22,23,24] The nausea and vomiting incidences in our clinic were higher when compared to previous studies. Our results were 65.9% and 48.2%, respectively. In previous studies, it has been reported that nausea and vomiting might form cluster separately as well as it might cluster along with lack of energy, lack of appetite and changes in the way food taste[9,25] or with pain and lack of energy.[26,27]
The fourth cluster included shortness of breath, difficulty in swallowing, coughing, and changes in the way food taste. Localization of the primary cancer is responsible for the etiology of shortness of breath, coughing, and difficulty in swallowing as well as advanced stage illness. The range of shortness of breath altering during rest or effort is reported to be 14%–39%.[28] Dyspnea was found to be 27.9% during effort and as 52.8% at rest in another study.[22] In the present study, a rate of 62.4% of dyspnea was stated by the patients which was relatively quite high. The preponderance of lung cancer patients and high incidences of all other symptoms may also lead to the frequent occurrence of dyspnea in the present study. Furthermore, dyspnea is a subjective symptom; therefore, anxiety, irritability, or pain may be sometimes expressed as dyspnea. Taste changes are more likely to be with gastrointestinal symptoms.[25] It is considered that the existence of change in the way food taste in this group was associated with the use of supplemental oxygen, opioid drugs, and inhaled medications.
The association of the symptoms forming the second cluster (feeling drowsy, numbness/tingling in hands/feet, difficulty in sleeping, dizziness, constipation, I do not look like myself) and the fifth cluster (feeling bloated, problems with urination, diarrhea, itching, mouth sores, hair loss, swelling of arm or legs, change in the skin) could not be interpreted. The symptoms seen in cancer patients may be related with the disease itself as well as the side effects of the treatment and comorbid conditions. Terminal stage-specific symptoms may be seen at the end of life.[2] Multifactorial structure affecting the experience of symptom makes the studies in this area difficult. The methodological and statistical differences of previous studies also make the comparison difficult.
In our study, gender was found to be effective on the physical symptom subscale. Walsh et al. found that gender affects symptoms significantly;[29] Homsi et al. found no significance.[30] Gender difference in the physical symptom may also be explained by the differences biological and sociological differences, females and males or observation that females report a greater symptom burden.
Limitations
In our study, many symptoms were investigated, and we encountered various symptom clusters in advanced stage cancer patients. We have used a 32-item scale that was validated for Turkish population. Although this may strengthen our study, we also have several limitations. We did not assess the prognostic effect of symptom clusters on survival or relationship with the primary site of the cancer. We think that long-term studies with large heterogeneous cancer groups are needed.
Conclusion
Identifying symptom clusters in oncological population according to cancer types particularly leads to changes in diagnostic criteria and contributes individualization of the treatment. However, the relationship of the symptoms observed in advanced cancer patients with type of cancer is gradually decreasing; therefore, it is possible to see different symptom clusters. Knowledge of cluster composition and their associations with quality of life and function is vital in the management of symptom clusters to improve patient outcomes. Our hospital is an oncology hospital, and we give supportive care to cancer patients in palliative care clinic. Palliative care is to be integrated into health care organization by new directives in our country. Although future studies are needed, we believe that the results of the present study may be useful in creating palliative care algorithms.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Dodd MJ, Miaskowski C, Paul SM. Symptom clusters and their effect on the functional status of patients with cancer. Oncol Nurs Forum. 2001;28:465–70. [PubMed] [Google Scholar]
- 2.Cheung WY, Barmala N, Zarinehbaf S, Rodin G, Le LW, Zimmermann C. The association of physical and psychological symptom burden with time to death among palliative cancer outpatients. J Pain Symptom Manage. 2009;37:297–304. doi: 10.1016/j.jpainsymman.2008.03.008. [DOI] [PubMed] [Google Scholar]
- 3.Dodd M, Janson S, Facione N, Faucett J, Froelicher ES, Humphreys J, et al. Advancing the science of symptom management. J Adv Nurs. 2001;33:668–76. doi: 10.1046/j.1365-2648.2001.01697.x. [DOI] [PubMed] [Google Scholar]
- 4.Esther Kim JE, Dodd MJ, Aouizerat BE, Jahan T, Miaskowski C. A review of the prevalence and impact of multiple symptoms in oncology patients. J Pain Symptom Manage. 2009;37:715–36. doi: 10.1016/j.jpainsymman.2008.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Miaskowski C. Symptom clusters: Establishing the link between clinical practice and symptom management research. Support Care Cancer. 2006;14:792–4. doi: 10.1007/s00520-006-0038-5. [DOI] [PubMed] [Google Scholar]
- 6.Yildirim Y, Tokem Y, Bozkurt N, Fadiloglu C, Uyar M, Uslu R. Reliability and validity of the Turkish version of the Memorial Symptom Assessment Scale in cancer patients. Asian Pac J Cancer Prev. 2011;12:3389–96. [PubMed] [Google Scholar]
- 7.Klinkenberg M, Willems DL, van der Wal G, Deeg DJ. Symptom burden in the last week of life. J Pain Symptom Manage. 2004;27:5–13. doi: 10.1016/j.jpainsymman.2003.05.008. [DOI] [PubMed] [Google Scholar]
- 8.Grunberg SM. New directions in supportive care. Support Care Cancer. 2005;13:135–7. doi: 10.1007/s00520-004-0742-y. [DOI] [PubMed] [Google Scholar]
- 9.Jiménez A, Madero R, Alonso A, Martínez-Marín V, Vilches Y, Martínez B, et al. Symptom clusters in advanced cancer. J Pain Symptom Manage. 2011;42:24–31. doi: 10.1016/j.jpainsymman.2010.10.266. [DOI] [PubMed] [Google Scholar]
- 10.Tsai JS, Wu CH, Chiu TY, Chen CY. Significance of symptom clustering in palliative care of advanced cancer patients. J Pain Symptom Manage. 2010;39:655–62. doi: 10.1016/j.jpainsymman.2009.09.005. [DOI] [PubMed] [Google Scholar]
- 11.Stiel S, Matthies DM, Seuş D, Walsh D, Lindena G, Ostgathe C. Symptoms and problem clusters in cancer and non-cancer patients in specialized palliative care-is there a difference? J Pain Symptom Manage. 2014;48:26–35. doi: 10.1016/j.jpainsymman.2013.08.018. [DOI] [PubMed] [Google Scholar]
- 12.Fleishman SB. Treatment of symptom clusters: Pain, depression, and fatigue. J Natl Cancer Inst Monogr. 2004;32:119–23. doi: 10.1093/jncimonographs/lgh028. [DOI] [PubMed] [Google Scholar]
- 13.Kirkova J, Aktas A, Walsh D, Davis MP. Cancer symptom clusters: Clinical and research methodology. J Palliat Med. 2011;14:1149–66. doi: 10.1089/jpm.2010.0507. [DOI] [PubMed] [Google Scholar]
- 14.Aktas A, Walsh D, Rybicki L. Symptom clusters: Myth or reality? Palliat Med. 2010;24:373–85. doi: 10.1177/0269216310367842. [DOI] [PubMed] [Google Scholar]
- 15.Dong ST, Costa DS, Butow PN, Lovell MR, Agar M, Velikova G, et al. Symptom clusters in advanced cancer patients: An empirical comparison of statistical methods and the ımpact on quality of life. J Pain Symptom Manage. 2016;51:88–98. doi: 10.1016/j.jpainsymman.2015.07.013. [DOI] [PubMed] [Google Scholar]
- 16.Ahles TA, Ruckdeschel JC, Blanchard EB. Cancer-related pain – I.Prevalence in an outpatient setting as a function of stage of disease and type of cancer. J Psychosom Res. 1984;28:115–9. doi: 10.1016/0022-3999(84)90003-5. [DOI] [PubMed] [Google Scholar]
- 17.Donnelly S, Walsh D. The symptoms of advanced cancer. Semin Oncol. 1995;22(2 Suppl 3):67–72. [PubMed] [Google Scholar]
- 18.Dixon S, Esper P. In: Anorexia, cachexia, and nutritional support. Palliative Care Practices from A to Z for the Bedside Clinicians. Kuebler K, Esper P, editors. Pittsburgh, PA: ONS Press; 2002. pp. 13–22. [Google Scholar]
- 19.Walch D, Donnelly S, Rybicky M. Symptoms of advanced cancer. Support Cancer Care. 2000;8:175–9. doi: 10.1007/s005200050281. [DOI] [PubMed] [Google Scholar]
- 20.Waller A, Caroline N. Handbook of Palliative Care in Cancer. 2nd ed. Boston, MA: Butterworth-Heinemann; 2000. [Google Scholar]
- 21.Vainio A, Auvinen A. Prevalence of symptoms among patients with advanced cancer: An international collaborative study. Symptom Prevalence Group. J Pain Symptom Manage. 1996;12:3–10. doi: 10.1016/0885-3924(96)00042-5. [DOI] [PubMed] [Google Scholar]
- 22.Gómez-Batiste X, Porta-Sales J, Espinosa-Rojas J, Pascual-López A, Tuca A, Rodriguez J. Effectiveness of palliative care services in symptom control of patients with advanced terminal cancer: A spanish, multicenter, prospective, quasi-experimental, pre-post study. J Pain Symptom Manage. 2010;40:652–60. doi: 10.1016/j.jpainsymman.2010.02.026. [DOI] [PubMed] [Google Scholar]
- 23.Walsh D, Rybicki L. Symptom clustering in advanced cancer. Support Care Cancer. 2006;14:831–6. doi: 10.1007/s00520-005-0899-z. [DOI] [PubMed] [Google Scholar]
- 24.Cheung WY, Le LW, Zimmermann C. Symptom clusters in patients with advanced cancers. Support Care Cancer. 2009;17:1223–30. doi: 10.1007/s00520-009-0577-7. [DOI] [PubMed] [Google Scholar]
- 25.Gift AG, Jablonski A, Stommel M, Given CW. Symptom clusters in elderly patients with lung cancer. Oncol Nurs Forum. 2004;31:202–12. doi: 10.1188/04.ONF.202-212. [DOI] [PubMed] [Google Scholar]
- 26.Laird BJ, Scott AC, Colvin LA, McKeon AL, Murray GD, Fearon KC, et al. Pain, depression, and fatigue as a symptom cluster in advanced cancer. J Pain Symptom Manage. 2011;42:1–11. doi: 10.1016/j.jpainsymman.2010.10.261. [DOI] [PubMed] [Google Scholar]
- 27.Fan G, Filipczak L, Chow E. Symptom clusters in cancer patients: A review of the literature. Curr Oncol. 2007;14:173–9. doi: 10.3747/co.2007.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Reddy SK, Parsons HA, Elsayem A, Palmer JL, Bruera E. Characteristics and correlates of dyspnea in patients with advanced cancer. J Palliat Med. 2009;12:29–36. doi: 10.1089/jpm.2008.0158. [DOI] [PubMed] [Google Scholar]
- 29.Walsh D, Donnelly S, Rybicki L. The symptoms of advanced cancer: Relationship to age, gender, and performance status in 1,000 patients. Support Care Cancer. 2010;8:175–9. doi: 10.1007/s005200050281. [DOI] [PubMed] [Google Scholar]
- 30.Homsi J, Walsh D, Rivera N, Rybicki LA, Nelson KA, Legrand SB, et al. Symptom evaluation in palliative medicine: Patient report vs. systematic assessment. Support Care Cancer. 2006;14:444–53. doi: 10.1007/s00520-005-0009-2. [DOI] [PubMed] [Google Scholar]


