FIGURE 1.
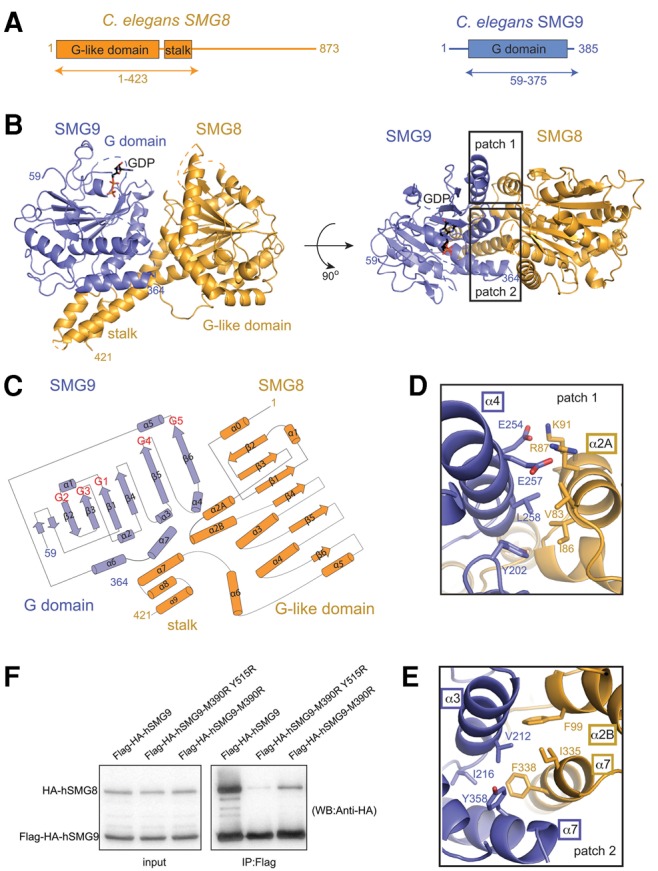
Structure of the conserved core of C. elegans SMG8–SMG9. (A) Schematic representation of the domain organization of C. elegans SMG8 (in orange) and SMG9 (in blue). Domains with a structured fold are shown as rectangles and labeled. Predicted low-complexity regions are shown as lines. The arrows below the diagram highlight the parts of the proteins that were crystallized. (B) Two views of the crystal structure of the C. elegans SMG8c–SMG9c core complex, with the molecules shown in orange and blue, respectively. The two views are related by a 90° clockwise rotation around a horizontal axis. The G-like domains and the stalk domain are indicated, as well as the N- and C-terminal residues with ordered electron density. The GDP moiety bound to the SMG9 G domain is shown in stick representation. Disordered loops are highlighted with dotted lines. On the right, the two rectangles highlight the two main interaction interfaces (patches 1 and 2) that are shown in more detail below in panels D and E. (C) Topological diagram of SMG8c and SMG9c (β-strands shown as arrows and α-helices as cylinders). Loops between secondary SMG9 feature similarities in the so-called G motifs as compared to other G domains. The positions of the G motifs in the loops between secondary structure elements are indicated in red. Note that SMG8c and SMG9c feature additional elements as compared to canonical G domains (α2A, α6 and α6, α7, respectively). (D) Zoomed-in view of the interacting residues at patch 1. The molecule is shown after ∼180° rotation around a horizontal axis with respect to the view in panel A. SMG8c helix α2A and SMG9c helices α3 and α4 are labeled. (E) Zoomed-in view of the interacting residues at patch 2. The molecule is shown in a similar orientation as in panel C. SMG8c stalk helices α2B and α7 and SMG9c helices α3 and α7 are indicated. (F) Coimmunoprecipitation assays of human full-length HA-tagged hSMG8 and Flag-HA-tagged hSMG9 (wild-type or mutants) in transiently transfected HEK293T cells. Cell lysates (input) were immunoprecipitated with Flag binder and detected with an HA-antibody (precipitate) (12% SDS-PAGE gel). The mutated residues in human SMG9 (M390 and Y515) correspond to C. elegans SMG9 Leu258 (patch 1, panel D) and Tyr358 (patch 2, panel E). HA-SMG8 is 111.7 kDa and Flag-HA-SMG9 is 63.6 kDa.
