Abstract
BACKGROUND
Treatment of multivessel coronary artery disease with traditional single-arterial coronary artery bypass graft (SA-CABG) has been associated with superior intermediate-term survival and reintervention compared with percutaneous coronary intervention (PCI) using either bare-metal stents (BMS) or drug-eluting stents (DES).
OBJECTIVES
This study sought to investigate longer-term outcomes including the potential added advantage of multiarterial coronary artery bypass graft (MA-CABG).
METHODS
We studied 8,402 single-institution, primary revascularization, multivessel coronary artery disease patients: 2,207 BMS-PCI (age 66.6 ± 11.9 years); 2,381 DES-PCI (age 65.9 ± 11.7 years); 2,289 SA-CABG (age 69.3 ± 9.0 years); and 1,525 MA-CABG (age 58.3 ± 8.7 years). Patients with myocardial infarction within 24 h, shock, or left main stents were excluded. Kaplan-Meier analysis and Cox regression were used to separately compare 9-year all-cause mortality and unplanned reintervention for BMS-PCI and DES-PCI to respective propensity-matched SA-CABG and MA-CABG cohorts.
RESULTS
BMS-PCI was associated with worse survival than SA-CABG, especially from 0 to 7 years (p = 0.015) and to a greater extent than MA-CABG was (9-year follow-up: 76.3% vs. 86.9%; p < 0.001). The surgery-to-BMS-PCI hazard ratios (HR) were as follows: versus SA-CABG, HR: 0.87; and versus MA-CABG, HR: 0.38. DES-PCI showed similar survival to SA-CABG except for a modest 0 to 3 years surgery advantage (HR: 1.06; p = 0.615). Compared with MA-CABG, DES-PCI exhibited worse survival at 5 (86.3% vs. 95.6%) and 9 (82.8% vs. 89.8%) years (HR: 0.45; p <0.001). Reintervention was substantially worse with PCI for all comparisons (all p <0.001).
CONCLUSIONS
Multiarterial surgical revascularization, compared with either BMS-PCI or DES-PCI, resulted in substantially enhanced death and reintervention-free survival. Accordingly, MA-CABG represents the optimal therapy for multivessel coronary artery disease and should be enthusiastically adopted by multidisciplinary heart teams as the best evidence-based therapy.
Keywords: arterial grafting, coronary stents, myocardial revascularization, propensity matching
The choice of optimal coronary revascularization method, particularly in the case of multivessel coronary artery disease (CAD), is a vigorously debated question that is of considerable importance to patients, clinicians, regulatory agencies, as well as third-party payers (1–6). Despite multiple methodological drawbacks of the related comparative studies (7), the near-uniform equivalence of long-term survival (~5 years) with coronary artery bypass graft (CABG) or percutaneous coronary intervention (PCI) has resulted in a dramatic increase in the rate of PCI at the expense of a substantial decrease in CABG volumes. Over the past decade, this debate has intensified with the introduction of drug-eluting stents (DES) as the new standard of care for PCI given their well-documented reduced restenosis rates and associated need for target vessel revascularization as compared with the rates of restenosis and target vessel revascularization for bare-metal stents (BMS) (8). Counterintuitively, however, the lower target vessel revascularization rates have not resulted in improved long-term survival or myocardial infarction rates with DES in most trials (9). This is while a number of “real-world” observational studies in multivessel CAD patients have suggested improved long-term mortality with DES (e.g., 9–12).
Randomized controlled trials (1,5,6,13) and large observational studies (8–10,14,15) focusing on multi-vessel CAD have uniformly associated CABG with significantly less need for coronary reinterventions, and most suggest modestly enhanced intermediate survival for CABG versus either DES-PCI or BMS-PCI. Moreover, the magnitude of the CABG advantage seems to depend on the extensiveness of the coronary disease and is largest for higher complexity cases with intermediate or high SYNTAX (Synergy Between Percutaneous Coronary Intervention With TAXUS and Cardiac Surgery) scores (5,8). Notably, this reported superiority of CABG is based on predominantly “conventional” single arterial coronary artery bypass graft (SA-CABG) as the preferred surgical method. SA-CABG, achieved mostly with the left internal thoracic artery (LITA) to left anterior descending (LAD) graft plus additional vein grafts, is the most common form of bypass surgery, but it may likely represent suboptimal surgical strategy. Indeed, compelling evidence has rapidly accumulated over the past decade suggesting a second arterial graft (i.e., multiarterial coronary artery bypass graft [MA-CABG]), most commonly involving the right internal thoracic artery or radial artery, improves intermediate and long-term outcomes substantially compared with those of SA-CABG (16–29).
Presently, there are no studies comparing intermediate-to-late outcomes (>5 years) of the presumed best percutaneous approach, or DES-PCI, to that of the presumed optimal surgical revascularization strategy, or MA-CABG. We thus have studied the hypothesis that, in patients with multivessel CAD, MA-CABG surgery will substantially and significantly extend the mortality and reintervention outcomes advantage observed with conventional SA-CABG over that of PCI, irrespective of stent type (BMS or DES). If true, such a finding has the potential to substantially reshape the debate regarding the optimal choice for treatment of multivessel CAD.
METHODS
STUDY POPULATION
These patients were derived from prospectively collected cardiac surgery (1994 to 2011) and interventional cardiology (1998 to 2009) clinical databases at Mount Sinai Beth Israel Medical Center (New York, New York). These databases are collected and reported in accordance with the requirements of the New York State Department of Health Reporting Systems. The institutional review board approved this study. The informed consent requirement was waived.
DESIGN OVERVIEW
In this retrospective analysis, patients were included if undergoing their first, nonemergency, coronary revascularization (PCI or CABG) for multivessel CAD. All patients had significant LAD disease and, in cases of CABG, must have received a LITA-LAD graft and a minimum of 2 bypass grafts. PCI patients were included only if ≥1 stent was successfully implanted. Alternatively, patients were excluded from the study in cases of previous coronary revascularization by CABG (n = 1,236) or PCI (n = 2,327), single vessel disease (n = 4,014), no LAD disease (n = 2,462), cardiogenic shock (25 PCI, 7 CABG), any myocardial infarction within 1 day of intervention (729 PCI, 60 CABG), if left main coronary stenting in cases of PCI (n = 112), or no stent PCI (226 failed PCI, 494 balloon angioplasty). Study-eligible patients were identified from a pool of 16,732 unique patients (11,999 PCI and 4,733 isolated CABG with LITA-LAD graft) and divided into 4 treatment groups: BMS-PCI; DES-PCI; SA-CABG; and MA-CABG. PCI patients receiving both DES and BMS stents were included in DES-PCI cohort. All MA-CABG in this study received ≥1 radial artery grafts in addition to a LITA-LAD graft with or without additional vein grafts.
OUTCOMES AND FOLLOW-UP
All cause-mortality and repeat coronary reintervention (PCI or CABG) ≤9 years after index primary revascularization were analyzed. Late all-cause mortality data were secured at the institution’s patient follow-up and verified from recurrent twice annual (last in April 2012) queries of the U.S. Social Security Death Index database. Given the frequent use of planned staged PCI to treat multivessel CAD, the first planned reintervention was not considered an outcome event. A planned-staged PCI was defined as a nonemergency procedure occurring within 6 weeks of primary PCI and where new target vessels are revascularized. Note, in such patients, a second repeat PCI (third overall) was counted as a reintervention event.
STATISTICAL ANALYSIS
The 4 PCI and CABG treatment study cohorts exhibited substantial demographic and risk factor differences (Table 1) that confound between-groups outcome comparisons. As a primary approach to obtain risk-adjusted PCI versus CABG outcome comparisons, we used propensity score matching—applied to 2 treatment groups at a time—to derive balanced demographic and risk factor subcohorts. Specifically, 4 matched patient-pair groups (1 PCI, 1 CABG) were based on propensity score models calculated separately for BMS-PCI and SA-CABG, BMS-PCI and MA-CABG, DES-PCI and SA-CABG, and DES-PCI and MA-CABG.
TABLE 1.
Selected Patient Demographic, Comorbidity, and Operative/Procedural Data Compared Across the Baseline (Pre-Matching) PCI and CABG Subcohorts
| Coronary Revascularization Method
|
||||
|---|---|---|---|---|
| BMS-PCI (n = 2,207) |
DES-PCI (n = 2,381) |
SA-CABG (n = 2,289) |
MA-CABG (n = 1,525) |
|
| Categorical variables | ||||
|
| ||||
| Male | 62.9 | 63.4 | 66.4 | 80.3 |
| BMI category, kg/m2 | ||||
| <18 | 1.0 | 1.1 | 0.7 | 0.5 |
| 18–24.99 | 23.5 | 24.0 | 30.9 | 23.3 |
| 25–29.99 | 41.1 | 39.6 | 40.2 | 40.5 |
| 30–34.99 | 22.0 | 22.3 | 20.2 | 23.0 |
| 35–39.99 | 8.4 | 8.5 | 6.1 | 8.9 |
| ≥40 | 4.1 | 4.4 | 1.9 | 3.8 |
| Obese | 34.4 | 35.2 | 28.2 | 35.7 |
| CCS classification | ||||
| 1 | 2.0 | 0.6 | 2.0 | 2.2 |
| 2 | 20.9 | 30.1 | 4.5 | 7.1 |
| 3 | 51.4 | 64.6 | 30.9 | 36.9 |
| 4 | 25.0 | 3.2 | 62.7 | 53.8 |
| Previous MI (>1 day) | 36.7 | 19.4 | 55.6 | 44.7 |
| Hypertension | 60.1 | 62.0 | 74.7 | 62.5 |
| COPD | 7.1 | 3.5 | 32.3 | 17.4 |
| Diabetes | 33.8 | 39.5 | 39.8 | 37.0 |
| Congestive heart failure | 10.7 | 5.8 | 17.6 | 7.0 |
| Creatinine >2.5 mg/dl | 2.1 | 3.0 | 5.4 | 1.0 |
| Renal dialysis | 2.3 | 2.6 | 3.1 | 0.3 |
| 3-vessel disease | 47.9 | 51.8 | 83.3 | 90.0 |
| 2-vessel disease | 52.1 | 48.2 | 16.7 | 10.0 |
| center main disease | 4.7 | 5.5 | 33.3 | 28.4 |
| Elective | 43.4 | 60.1 | 22.1 | 24.2 |
| Urgent | 56.6 | 39.9 | 77.9 | 75.8 |
| Off-pump CABG | 4.0 | 1.8 | ||
| Outcomes | ||||
| Reintervention, ≥1 | 38.5 | 44.6 | 9.5 | 10.2 |
| In-hospital death | 0.5 | 0.2 | 1.7 | 0.3 |
|
| ||||
| Continuous variables | ||||
|
| ||||
| Age, yrs | 66.6 ± 11.9 | 65.9 ± 11.7 | 69.3 ± 9.0 | 58.3 ± 8.7 |
| Height, cm | 166 ± 10 | 166 ± 10 | 166 ± 10 | 170 ± 10 |
| Weight, kg | 79 ± 17 | 79 ± 18 | 77 ± 15 | 83 ± 17 |
| BMI, kg/m2 | 28.7 ± 5.6 | 28.8 ± 5.8 | 27.7 ± 5.0 | 28.9 ± 5.3 |
| BSA, m2 | 1.90 ± 0.24 | 1.90 ± 0.24 | 1.88 ± 0.22 | 1.97 ± 0.23 |
| Ejection fraction, % | 51 ± 12 | 54 ± 12 | 48 ± 13 | 51 ± 12 |
Values are % or mean ± SD.
BMI = body mass index; BMS = bare-metal stent(s); BSA = body surface area; CABG = coronary artery bypass graft; CCS = Canadian Cardiovascular Society; COPD = chronic obstructive pulmonary disease; DES = drug-eluting stent(s); MA = multiarterial; MI = myocardial infarction; PCI = percutaneous coronary intervention; SA = single-arterial.
All propensity models considered PCI as treatment for the calculation of probability of PCI via non-parsimonious logistic multivariate models using 21 demographics and preoperative risk factors. Left main disease was not included in the propensity model given its relative rarity in PCI-treated patients versus CABG-treated (5% vs. 32%) so as not to severely limit the matching of patients. A 1-to-1 greedy propensity score matching was obtained in each case and always to the closest available score and within ±1% difference. Patient factors for matched patient groups were compared to ensure matching adequacy defined as standardized differences <10% for all continuous and categorical variables (30).
Continuous data were expressed as mean ± SD. When applicable, univariate comparisons were done with chi-square or Fisher exact tests for categorical variables and the unpaired Student t test or the nonparametric Mann-Whitney U test for continuous variables. All-cause mortality and reintervention-free survival comparisons were done via Kaplan-Meier analysis (log-rank test) on the matched subcohorts. The corresponding risk-adjusted hazard ratios (HR) (with and without 95% confidence intervals [CIs]) relating the different treatments, including the potential residual effect of left main disease (not included in propensity models), were estimated by proportional hazard Cox regression for the entire 9-year follow-up as a form of time-averaged treatment effect. Additionally, given evidence of non-proportional hazards for the entire 9-year follow-up period, we repeated a time-segmented Cox regression based on time cutoffs of 3 and 4.5 years for SA-CABG and MA-CABG, respectively. Lastly, a confirmatory analysis was done in all patients to ascertain the HR for the same pairwise comparisons using comprehensive multivariate risk adjustment via Cox regression (Table 1). A 2-sided p value of <0.05 was used to indicate significance. Statistical analysis was conducted with SPSS software (version 22, IBM, Armonk, New York).
RESULTS
A total of 8,402 multivessel CAD patients undergoing their primary revascularization were study-eligible and distributed as follows: BMS-PCI (n = 2,207; age 66.6 ± 11.9 years); DES-PCI (n = 2,381; age 65.9 ± 11.7 years); SA-CABG (n = 2,289; age 69.3 ± 9.0 years); and MA-CABG (n = 1,525; age 58.3± 8.7 years). Patient characteristics differed substantially for the different revascularization method cohorts (Table 1). Notably, MA-CABG patients were younger and more were male (80.3%), reflecting a practice selection; however, this has changed over the study period with both the median age (57.2 vs. 61.5 years) and proportion of women (17.7% vs. 24.7%) increasing between 1995 to 1996 and 2009 to 2011, respectively.
PCI TRENDS
First coronary interventions amounted to 77% (9,242 of 11,999) of all unique PCI patients, whereas remaining patients had previous PCI (n = 1,526; 12.7%), CABG (n = 915; 7.6%), or both (n = 292; 2.4%) and thus were excluded from the analysis. A total of 4,588 (49.6%) of the primary (first-time) PCI patients met the inclusion criteria. The latter increased systematically over the study period (36% [1998] up to 60% [2008 to 2009]) (Online Figure 1) and reflected a trend of more frequent use of PCI as a primary modality to treat multivessel CAD. BMS were used exclusively before 2003, whereas DES-PCI increased progressively after that to about 85% to 90% of all stent PCI by 2007 to 2009 (Online Figure 2). The DES cohort in the present study included 1,833 first-generation (77%; 1:2 sirolimus to paclitaxel) and 548 second-generation (23%; 5:1 zotarolimus to everolimus) DES.
UNADJUSTED OUTCOMES
The unadjusted in-hospital mortality was comparable for BMS-PCI versus DES-PCI (12 of 2,207 [0.54%] vs. 4 of 2,381 [0.17%]; p = 0.057), but it was distinctly lower for the younger and healthier MA-CABG patients versus SA-CABG patients (4 of 1,525 [0.26%] vs. 38 of 2,289 [1.66%]; p < 0.001). Unadjusted Kaplan-Meier death-free survival differed substantially for the 4 treatment groups (p < 0.001 overall and pairwise) (Figure 1A). Here, MA-CABG (mean age: 58 years) exhibited the best 9-year survival and SA-CABG (69 years), the worst survival, whereas PCI patients showed intermediate survival with DES-PCI superior to survival with BMS-PCI.
FIGURE 1. Survival After Percutaneous and Surgical CAD Treatment Modalities.
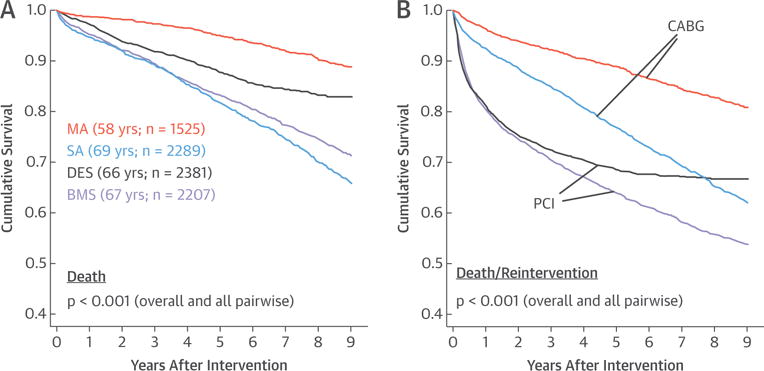
Comparisons of unadjusted 9-year all-cause mortality (A) and unplanned reintervention-free (B) survival shown for all 4 coronary revascularization groups: 2,207 bare-metal stent (BMS) percutaneous coronary intervention (PCI) (age 66.6 ± 11.9 years); 2,381 drug-eluting stent (DES)-PCI (age 65.9 ± 11.7 years); 2,289 single-arterial (SA) coronary artery bypass graft (CABG) (age 69.3 ± 9.0 years); and 1,525 multiarterial (MA)-CABG (age 58.3 ± 8.7 years). The p values were derived by log-rank test. CAD = coronary artery disease.
Planned PCI were frequently used as part of a staged treatment approach given their multivessel disease (1,060 of 4,588; 23.1% overall), and this was more prevalent with the more recent DES-PCI (28.8% vs. 16.9%; p < 0.001). In contrast, unplanned reinterventions (PCI or CABG) were more frequent in BMS-PCI (21.5% vs. 15.8%; p < 0.001), but this may partly reflect their relatively longer follow-up. Both CABG modalities were associated with superior unadjusted, unplanned reintervention-free survival compared with rates for both BMS and DES groups, which had essentially identical 0 to 3 years unplanned reintervention outcomes (Figure 1B). Time-to-reintervention analysis showed a distinct change in the relative proportions and time course of the planned versus unplanned reintervention in BMS-PCI versus DES-PCI (Figures 2A and 2B). The relative decrease in unplanned reinterventions with DES probably reflects their superiority over BMS, whereas the increased planned PCI among DES reflect the changing practice patterns of interventional cardiologists in more recent years toward percutaneous treatment of more complex disease requiring multiple PCI.
FIGURE 2. 9-Year Mortality and Reintervention Outcomes for BMS- Versus DES-PCI.
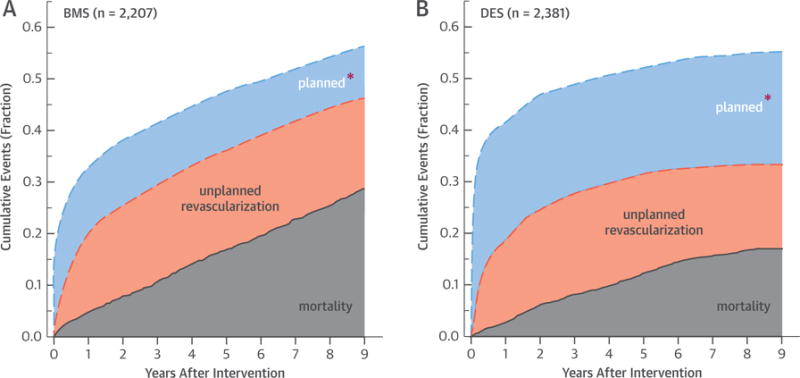
Breakdown of 9-year cumulative event rates to their all-cause mortality, planned reinterventions, and unplanned reinterventions for multivessel coronary artery disease patient cohorts treated with intracoronary stenting at their index (first) revascularization procedure: BMS-PCI cohort (A) and DES-PCI cohort (B). *Definition of planned PCI provided under Outcomes and Follow-up in the Methods section. Abbreviations as in Figure 1.
MATCHED-ADJUSTED COMPARISONS
The number of matched patients, based on separate propensity score models, differed for the 4-pairwise comparisons: BMS-PCI versus SA-CABG (1,058 pairs); BMS-PCI versus MA-CABG (746 pairs); DES-PCI versus SA-CABG (667 pairs); and DES-PCI versus MA-CABG (546 pairs). Matching successfully identified comparison subcohorts with similar demographics, risk factors, and comorbidities (Online Tables 1 to 4).
BMS-PCI was associated with worse death-free survival than SA-CABG was, especially for the first 7 years of follow-up (p = 0.015; mean age = 68 years; 73% 3-vessel disease) (Central Illustration), and to a greater extent when compared with survival rates for MA-CABG, which showed a 10% absolute BMS-PCI versus MA-CABG difference at 5 (85.2% vs. 95.0%) and 9 (76.3% vs. 86.9%) years (p < 0.001; mean age = 61 years; 82% 3-vessel disease) (Central Illustration). Alternatively, DES-PCI showed similar death-free survival as for SA-CABG, except for a modest SA-CABG advantage for the first 3 years (p = 0.615; mean age = 68 years; 78% 3-vessel disease) (Central Illustration). Lastly, DES-PCI exhibited worse mortality than MA-CABG did with death-free survival of 86.3% versus 95.6% at 5 years and 82.8% versus 89.8% at 9 years (p < 0.001; mean age = 60.4 years; 84% 3-vessel disease) (Central Illustration). Reintervention-free survival was substantially and significantly worse with PCI irrespective of stent type when compared with survival rates for either single arterial or multi-arterial CABG (Figures 3A to 3D). The corresponding 9-year mortality HR estimates are summarized in Figure 4. Briefly, the surgery-to-BMS-PCI HR were in favor of CABG surgery approaching statistical significance in cases of SA-CABG (HR: 0.87; 95% CI: 0.75 to 1.02; p = 0.056) and were substantial and highly significant for MA-CABG versus BMS-PCI (HR: 0.38; 95% CI: 0.31 to 0.46; p < 0.001). Alternatively, DES-PCI and SA-CABG 9-year survival was comparable with a near-unity HR (HR: 1.06; 95% CI: 0.85 to 1.32; p = 0.615). Last, the MA-CABG versus DES-PCI survival advantage was substantial resulting in an HR of 0.45 (95% CI: 0.31 to 0.66) over the 9-year follow-up period (p < 0.001). These 9-year hazard estimates were essentially unchanged when further adjusted for the presence of left main disease and were also similar when derived by comprehensive risk-adjustment in all versus matched-only patients (Figure 4). Lastly, all 4 survival comparisons in propensity matched subcohorts (Central Illustration) exhibited significant time modification (interaction term) of the early versus late HR. Specifically, an early advantage of SA-CABG ≤3 years was lost when compared with the advantage of BMS, whereas it was completely reversed versus the advantage of DES. In case of MA-CABG, a more substantial advantage over either BMS or DES was sustained for a longer period (4.5 years) and was not reversed even if it lost significance (Table 2).
CENTRAL ILLUSTRATION. Optimal Revascularization of Multivessel CAD: Comparison of 9-Year Propensity Matched All-Cause Mortality Survival Data for Both PCI Treatment Cohorts.
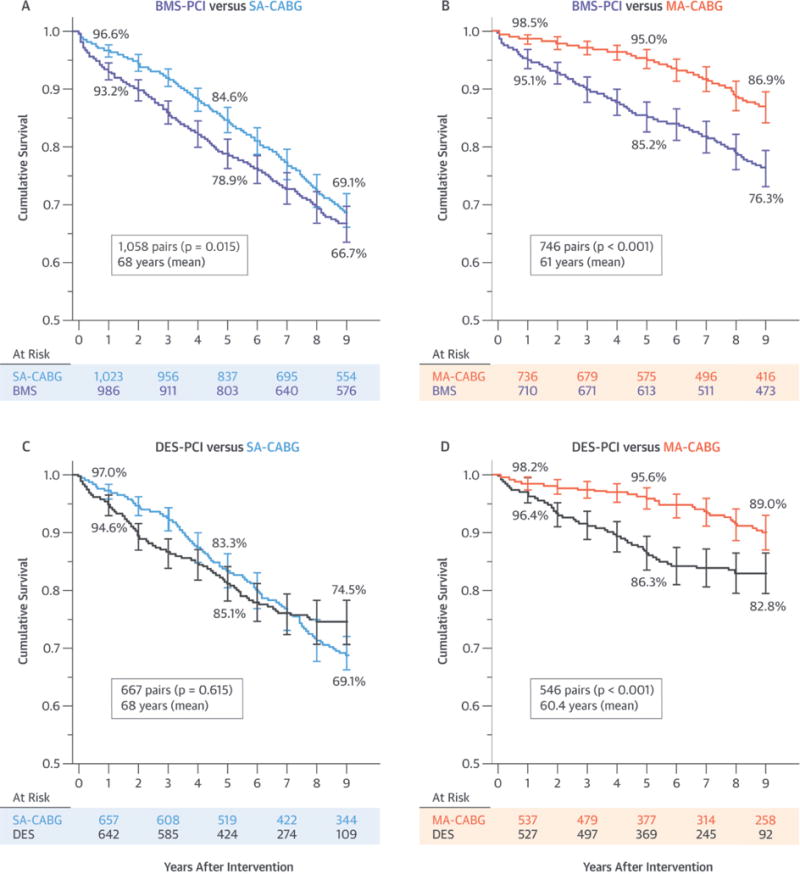
Each cohort is separately compared to single-arterial (SA) and multiarterial (MA) coronary artery bypass graft (CABG) surgery: (A) bare-metal stent (BMS) percutaneous coronary intervention (PCI) versus SA-CABG; (B) BMS-PCI versus MA-CABG; (C) drug-eluting stent (DES)-PCI versus SA-CABG; and (D) DES-PCI versus MA-CABG. The p values were derived by log-rank test. CAD = coronary artery disease.
FIGURE 3. Pairwise PCI Versus CABG Comparisons of Match-Adjusted Unplanned Reintervention.
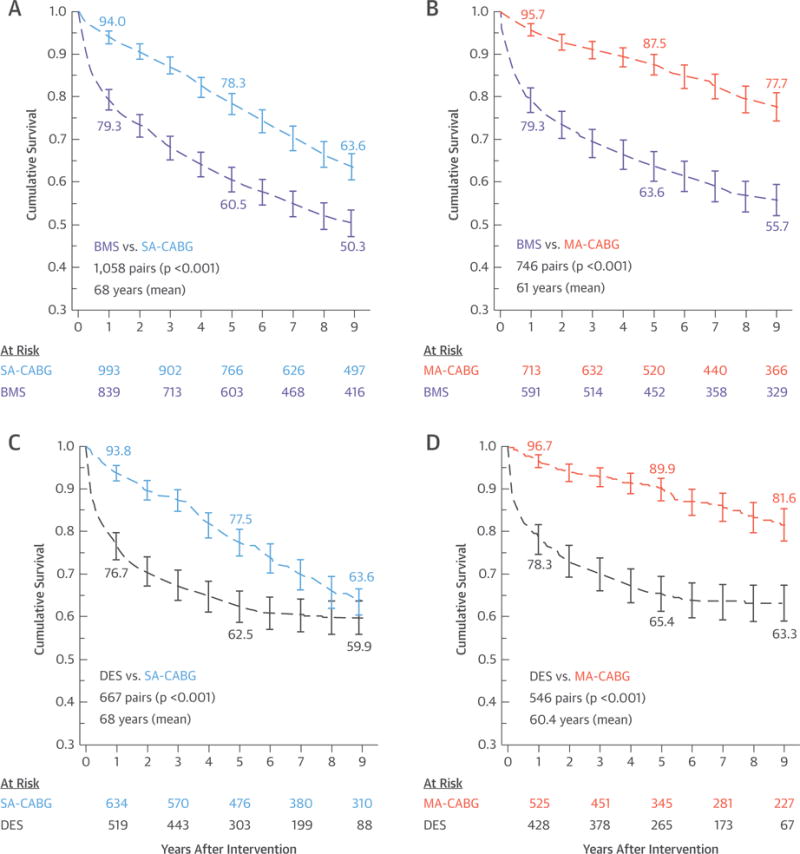
Comparison of 9-year propensity-matched reintervention-free survival data for both PCI treatment cohorts with each separately compared with SA-CABG and MA-CABG surgery: (A) BMS-PCI versus SA-CABG; (B) BMS-PCI versus MA-CABG; (C) DES-PCI versus SA-CABG; and (D) DES-PCI versus MA-CABG. The p values were derived by log-rank test. Abbreviations as in Figure 1.
FIGURE 4. Pairwise Adjusted Hazard Ratios of CABG Versus PCI Modalities.
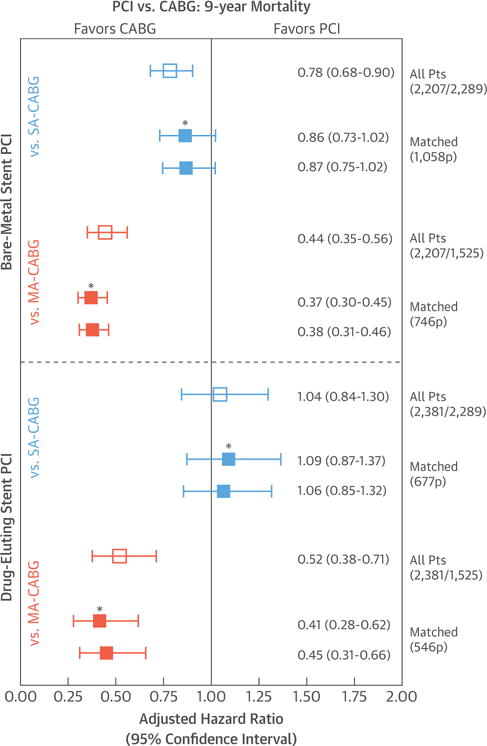
Risk-adjusted 9-year all-cause mortality CABG-to-PCI hazard ratios derived for both the BMS-PCI and DES-PCI treatment cohorts when each is compared with the SA-CABG and MA-CABG surgical treatments. Solid squares reflect hazard ratios derived in matched patient cohorts (*additional adjustment for left main disease, which was not included in propensity models). Open squares reflect hazard ratios derived from all available patients using forced risk-adjustments (22 factors) via proportional hazard Cox regression. Pts = patients; other abbreviations as in Figure 1.
TABLE 2.
Time-Segmented HR Derived by Time-Dependent Covariate Cox Regression Comparing PCI Modalities (BMS or DES) to SA- and MA-CABG Surgery
| HR (95% CI) | p Value | |
|---|---|---|
| SA-CABG vs. BMS | ||
| <3 yrs | 0.55 (0.42–0.72) | <0.001 |
| >3 yrs | 1.12 (0.93–1.37) | 0.240 |
| Time*Treatment | <0.001 | |
|
| ||
| SA-CABG vs. DES | ||
| <3 yrs | 0.54 (0.38–0.76) | <0.001 |
| >3 yrs | 1.79 (1.32–2.43) | <0.001 |
| Time*Treatment | <0.001 | |
|
| ||
| MA-CABG vs. BMS | ||
| <4.5 yrs | 0.29 (0.19–0.43) | <0.001 |
| >4.5 yrs | 0.82 (0.56–1.19) | 0.290 |
| Time*Treatment | <0.001 | |
|
| ||
| MA-CABG vs. DES | ||
| <4.5 yrs | 0.28 (0.17–0.47) | <0.001 |
| >4.5 yrs | 0.98 (0.53–1.80) | 0.936 |
| Time*Treatment | 0.002 | |
CI = confidence interval; HR = hazard ratio; Time*Treatment = interaction term defining the time-varying treatment effect in each of the 4 comparisons’ Cox regression analysis; other abbreviations as in Table 1.
DISCUSSION
Identifying the optimal revascularization strategy for multivessel CAD necessarily requires a direct comparison of outcomes of the most durable surgical technique to the best percutaneous approach. Thus, based on current evidence, an appropriately designed analysis comparing outcomes of MA-CABG to DES-PCI is needed. To our knowledge, this has not been yet reported. This study is the first to specifically compare long-term survival and reintervention rates in multivessel CAD revascularized either via the traditional single-arterial (LITA-LAD graft) or multi-arterial (95% LITA and radial artery in the current series) CABG versus PCI with either BMS or DES. The primary finding was that MA-CABG, compared with either BMS- or DES-PCI, results in substantially enhanced death-free and reintervention-free survival and, therefore, represents optimal therapy. Secondary study findings confirm previously reported observations that conventional SA-CABG is associated with better intermediate-term survival, and superior reintervention rates compared with those for BMS (1,3,8,9). In addition, conventional SA-CABG is associated with better intervention-free survival than PCI-DES is, whereas the 2 methods showed similar late death-free survival following a brief trend (0 to 3 years) of SA-CABG superiority (Central Illustration).
Multiple randomized prospective studies were published in the past decade comparing surgical approaches versus percutaneous approaches (1–6). Their results mostly showed equivalent long-term survival with both modalities albeit at the expense of more frequent reintervention, yet these had limited applicability to the “real-world” scenarios confronting clinicians given the relatively healthy and highly selective nature of these trials’ populations (7,31). Furthermore, these studies uniformly compared only traditional SA-CABG with BMS-PCI or DES-PCI. Our analysis results contradicted these trials and, instead, were consistent with findings of more recent large real-world observational retrospective studies (8–15).
The ASCERT (ACCF-STS Database Collaboration on the Comparative Effectiveness of Revascularization Strategies) trial linked the Society of Thoracic Surgeons Adult Database and the NCDR (National Cardiovascular Data Registry) National Database with the Centers for Medicare and Medicaid Services Administrative Database and used propensity score methods (inverse probability weighting) to assess long-term outcomes in Medicare beneficiaries treated with either CABG or PCI (14). In their PCI cohort, 78% received DES, 16% received BMS, and 6% received no stents. Use of multiple arterial graft in ASCERT was not reported, but given the 10% overall reported rate of MA-CABG in the Society of Thoracic Surgeons’ database, logic dictates that the rate of MA-CABG is likely in the single digits, given the propensity to use MA-CABG in young or non-Medicare patients. This study principally comparing SA-CABG to predominantly DES-PCI reported an adjusted 4-year mortality of 16.4% with CABG versus 20.8% for PCI (HR: 0.79; 95% CI: 0.76 to 0.82). We, in the general primary intervention multivessel CAD population, found a modest early-to-intermediate-term survival advantage of conventional CABG over DES-PCI that is essentially eliminated by the fourth year after the index revascularization (Central Illustration). Given this result, a preference of DES-PCI is arguably a reasonable treatment choice, especially if the risk of other major adverse cardiovascular and cerebrovascular events is not meaningfully increased. This is true even acknowledging the formidable likelihood of future repeat PCI in multivessel CAD patients, which would then be weighed against the greater immediate potential of morbidity and longer recovery after CABG. From this perspective, our data support the point of view of a large segment of cardiologists that DES-PCI is an attractive alternative to traditional CABG surgery even in multivessel CAD patients. A salient counterargument, however, is the concern over the cost-effectiveness concern of 1 or more repeat DES-PCI reinterventions in a time of increasingly scarce available health care resources (32).
The novel finding of the current study is that MA-CABG is associated with large death-free and reintervention-free survival advantages over BMS and more pertinently DES-PCI (Central Illustration, Figures 3 and 4). This unique and important finding represents a potentially game-changing perspective to the ongoing PCI versus CABG debate. Specifically, the benefits attributable to MA-CABG are substantially greater than those previously reported with SA-CABG. Moreover, the dramatic reintervention-free advantage with MA-CABG versus DES-PCI is found even when a large number of planned repeat DES-PCI procedures are considered as part of the overall PCI treatment rather than reintervention events or outcomes (Figure 2). Ironically, the apparent reluctance of cardiac surgeons to embrace MA-CABG, despite the strong and rapidly accumulating evidence in its favor, lends further support to the point of view of favoring DES-PCI in a large majority of CAD patients. This stance by interventional cardiologists appears to have been reinforced by the more favorable results of DES and is evidenced in the rapid increase of PCI in multivessel CAD patients (Online Figure 1) even if it means more frequent use of planned, staged PCI treatments (i.e., multiple PCI procedures) as a means of achieving more complete revascularization (Figure 2).
The explanation of why MA-CABG resulted in superior intermediate and late mortality outcomes compared with those of DES-PCI while SA-CABG did not is likely due to the better patency of arterial grafts versus saphenous vein grafts (17,33,34). More complete, or even supra-complete (or functional revascularization), compared with PCI has been suggested as a reason for better CABG outcomes (23,25,26). However, this is equally true with either CABG modality and cannot explain our mortality data. It is, however, consistent with the comparably reduced need for reintervention with either form of CABG compared with either form of PCI. Hence, our findings, may indicate potentially superior patency of arterial grafts compared with those of DES and certainly those of BMS. Patency of vein grafts may not be superior to DES and, hence, the essentially equivalent survival outcomes in our study and in most studies comparing DES to traditional SA-CABG (1–6,33,34). The recent literature addressing the superior late outcomes of multiarterial versus traditional CABG has put forth the decreased progression of native vessel disease in coronary territories revascularized with arterial grafts compared with vein grafts as an explanatory factor (35). This finding was based on a review of a large number of coronary recatheterization data in CABG-treated patients, and relatively greater release of nitric oxide from arterial versus vein graft tissues has been suggested as the mechanism for this protection of native coronary beds against progression of atherosclerosis (35,36).
STUDY LIMITATIONS
Our study findings are based on a retrospective analysis of PCI and CABG data from a single institution, which may limit their generalizability. Propensity matching may not have accounted for unmeasured confounders. The matched MA-CABG versus PCI comparisons were in relatively young (60 to 61 years, mean age) patients and may not reflect results in older patients. Related to this, it is not clear whether the current findings will be similarly true across various risk-based or demographic subcohorts of the general multivessel CAD population. This concern is partly mitigated by our secondary Cox regression analysis applied to all patients as opposed to the matched cohorts only, which showed relatively similar comparative findings and similar risk-adjusted HR (Figure 4). Our mortality analysis was an all-cause deaths analysis, and we could not ascertain cardiac-related deaths. Yet, the similarity of the patient characteristics following propensity matching may partially mitigate this concern. It is possible that, within the characteristics of the included patients, the various reported PCI versus CABG outcomes comparison could differ appreciably for different demographic (age, sex, body mass index) or risk factor/comorbidity (diabetes, left ventricular dysfunction) subcohorts. These risk-based subcohort analyses are, however, well outside the scope of the current study and should be the focus of future efforts. Similarly, an analysis of the health economics implications of the different treatment options seems warranted especially given the apparently frequent use of repeat planned and unplanned PCI reinterventions. We also cannot be completely certain of the degree of functional completeness of revascularization in either the PCI or CABG cohorts. Differences in post-discharge medical therapy may have differed across study groups. In the current CAD series, all CABG and PCI patients were discharged from the hospital on standard guideline-directed medical therapy. Compliance with the guidelines during hospitalization and upon discharge is tracked by the hospital and by the New York State Department of Health, but we do not have post-discharge or long-term compliance data. SYNTAX scores are unavailable for study patients; hence, the precise distribution of the severity of the CAD in the different comparison groups is not known. Yet, our study included multivessel CAD patients and all with LAD disease exclusively, and all comparisons were propensity matched for 2- and 3-vessel disease as a means of minimizing the heterogeneity. Furthermore, we conducted a sensitivity analysis in which we further adjusted for left main disease, which showed consistent results (Figure 4).
CONCLUSIONS
The collective findings in our study, if confirmed in other series, have the potential to drastically modify the PCI versus CABG debate. Multiarterial surgical revascularization—shown previously to be superior to SA-CABG (16–29)—resulted in substantially enhanced death and reintervention-free survival compared with survival rates for either BMS- or DES-PCI. Accordingly, MA-CABG represents the optimal therapy for multivessel CAD and should be enthusiastically adopted by practicing cardiac surgeons and members of the multidisciplinary heart team as they strive to implement best evidence-based therapy.
Supplementary Material
PERSPECTIVES.
COMPETENCY IN MEDICAL KNOWLEDGE
In patients with multivessel CAD, intermediate-to-late mortality and need for subsequent revascularization are reduced when multiple arterial grafts are employed during CABG versus single arterial or percutaneous revascularization.
TRANSLATIONAL OUTLOOK
Future investigation is needed to enhance the selection of patients with multivessel CAD who benefit most from MA-CABG surgery.
Acknowledgments
This research was funded by departmental and institutional funds. Drs. Badour, Yammine, and El-Hage-Sleiman are funded in part by National Institutes of Health Training Grant #1D43TW009118-01A1 awarded to Scholars in Health Research Program, American University of Beirut, Beirut, Lebanon.
ABBREVIATIONS AND ACRONYMS
- BMS
bare-metal stent(s)
- CABG
coronary artery bypass graft
- CAD
coronary artery disease
- CI
confidence interval
- DES
drug-eluting stent(s)
- HR
hazard ratio
- LAD
left anterior descending
- LITA
left internal thoracic artery
- MA-CABG
multiarterial coronary artery bypass graft
- PCI
percutaneous coronary intervention
- SA-CABG
single-arterial coronary artery bypass graft
APPENDIX
For supplemental tables and figures, please see the online version of this article.
Footnotes
The authors have reported that they have no relationships relevant to the contents of this paper to disclose. Richard Shemin, MD, served as Guest Editor for this paper.
Presented in part as an oral Plenary Session presentation at the American Association for Thoracic Surgeons Meeting, April 26 to 30, 2014, Toronto, Canada.
Listen to this manuscript’s audio summary by JACC Editor-in-Chief Dr. Valentin Fuster.
References
- 1.Serruys PW, Ong AT, van Herwerden LA, et al. Five-year outcomes after coronary stenting versus bypass surgery for the treatment of multivessel disease: the final analysis of the Arterial Revascularization Therapies Study (ARTS) randomized trial. J Am Coll Cardiol. 2005;46:575–81. doi: 10.1016/j.jacc.2004.12.082. [DOI] [PubMed] [Google Scholar]
- 2.Hueb W, Lopes NH, Gersh BJ, et al. Five-year follow-up of the Medicine, Angioplasty, or Surgery Study (MASS II): a randomized controlled clinical trial of 3 therapeutic strategies for multivessel coronary artery disease. Circulation. 2007;115:1082–9. doi: 10.1161/CIRCULATIONAHA.106.625475. [DOI] [PubMed] [Google Scholar]
- 3.Booth J, Clayton T, Pepper J, et al. for the SoS Investigators Randomized, controlled trial of coronary artery bypass surgery versus percutaneous coronary intervention in patients with multivessel coronary artery disease: six-year follow-up from the Stent or Surgery Trial (SoS) Circulation. 2008;118:381–8. doi: 10.1161/CIRCULATIONAHA.107.739144. [DOI] [PubMed] [Google Scholar]
- 4.Kapur A, Hall RJ, Malik IS, et al. Randomized comparison of percutaneous coronary intervention with coronary artery bypass grafting in diabetic patients: 1-year results of the CARDia (Coronary Artery Revascularization in Diabetes) trial. J Am Coll Cardiol. 2010;55:432–40. doi: 10.1016/j.jacc.2009.10.014. [DOI] [PubMed] [Google Scholar]
- 5.Kappetein AP, Feldman TE, Mack MJ, et al. Comparison of coronary bypass surgery with drug-eluting stenting for the treatment of left main and/or three-vessel disease: 3-year follow-up of the SYNTAX trial. Eur Heart J. 2011;32:2125–34. doi: 10.1093/eurheartj/ehr213. [DOI] [PubMed] [Google Scholar]
- 6.Farkouh ME, Domanski M, Sleeper LA, et al. for the FREEDOM Trial Investigators Strategies for multivessel revascularization in patients with diabetes. N Engl J Med. 2012;367:2375–84. doi: 10.1056/NEJMoa1211585. [DOI] [PubMed] [Google Scholar]
- 7.Taggart DP. Thomas B. Ferguson Lecture. Coronary artery bypass grafting is still the best treatment for multivessel and left main disease, but patients need to know. Ann Thorac Surg. 2006;82:1966–75. doi: 10.1016/j.athoracsur.2006.06.035. [DOI] [PubMed] [Google Scholar]
- 8.Hannan EL, Wu C, Walford G, et al. Drug-eluting stents vs. coronary-artery bypass grafting in multivessel coronary disease. N Engl J Med. 2008;358:331–41. doi: 10.1056/NEJMoa071804. [DOI] [PubMed] [Google Scholar]
- 9.Hannan EL, Racz MJ, Walford G, et al. Long-term outcomes of coronary-artery bypass grafting versus stent implantation. N Engl J Med. 2005;352:2174–83. doi: 10.1056/NEJMoa040316. [DOI] [PubMed] [Google Scholar]
- 10.Wu C, Camacho FT, Zhao S, et al. Long-term mortality of coronary artery bypass graft surgery and stenting with drug-eluting stents. Ann Thorac Surg. 2013;95:1297–305. doi: 10.1016/j.athoracsur.2012.11.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Spaulding C, Daemen J, Boersma E, Cutlip DE, Serruys PW. A pooled analysis of data comparing sirolimus-eluting stents with bare-metal stents. N Engl J Med. 2007;356:989–97. doi: 10.1056/NEJMoa066633. [DOI] [PubMed] [Google Scholar]
- 12.Natsuaki M, Morimoto T, Furukawa Y, et al. for the CREDO-Kyoto PCI/CABG Registry Cohort-2 Investigators Late adverse events after implantation of sirolimus-eluting stent and bare-metal stent: long-term (5–7 years) follow-up of the Coronary Revascularization Demonstrating Outcome study-Kyoto registry Cohort-2. Circ Cardiovasc Interv. 2014;7:168–79. doi: 10.1161/CIRCINTERVENTIONS.113.000987. [DOI] [PubMed] [Google Scholar]
- 13.Verma S, Farkouh ME, Yanagawa B, et al. Comparison of coronary artery bypass surgery and percutaneous coronary intervention in patients with diabetes: a meta-analysis of randomized controlled trials. Lancet Diabetes Endocrinol. 2013;1:317–28. doi: 10.1016/S2213-8587(13)70089-5. [DOI] [PubMed] [Google Scholar]
- 14.Weintraub WS, Grau-Sepulveda MV, Weiss JM, et al. Comparative effectiveness of revascularization strategies. N Engl J Med. 2012;366:1467–76. doi: 10.1056/NEJMoa1110717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim YG, Park DW, Lee WS, et al. Influence of diabetes mellitus on long-term (five-year) outcomes of drug-eluting stents and coronary artery bypass grafting for multivessel coronary revascularization. Am J Cardiol. 2012;109:1548–57. doi: 10.1016/j.amjcard.2012.01.377. [DOI] [PubMed] [Google Scholar]
- 16.Lytle BW, Blackstone EH, Sabik JF, Houghtaling P, Loop FD, Cosgrove DM. The effect of bilateral internal thoracic artery grafting on survival during 20 postoperative years. Ann Thorac Surg. 2004;78:2005–12. doi: 10.1016/j.athoracsur.2004.05.070. [DOI] [PubMed] [Google Scholar]
- 17.Zacharias A, Habib RH, Schwann TA, Riordan CJ, Durham SJ, Shah A. Improved survival with radial artery versus vein conduits in coronary bypass surgery with left internal thoracic artery to left anterior descending artery grafting. Circulation. 2004;109:1489–96. doi: 10.1161/01.CIR.0000121743.10146.78. [DOI] [PubMed] [Google Scholar]
- 18.Rankin JS, Tuttle RH, Wechsler AS, Teichmann TL, Glower DD, Califf RM. Techniques and benefits of multiple internal mammary artery bypass at 20 years of follow-up. Ann Thorac Surg. 2007;83:1008–14. doi: 10.1016/j.athoracsur.2006.10.032. [DOI] [PubMed] [Google Scholar]
- 19.Zacharias A, Schwann TA, Riordan CJ, Durham SJ, Shah AS, Habib RH. Late results of conventional versus all-arterial revascularization based on internal thoracic and radial artery grafting. Ann Thorac Surg. 2009;87:19–26.e2. doi: 10.1016/j.athoracsur.2008.09.050. [DOI] [PubMed] [Google Scholar]
- 20.Schwann TA, Zacharias A, Riordan CJ, Durham SJ, Shah AS, Habib RH. Sequential radial artery grafts for multivessel coronary artery bypass graft surgery: 10-year survival and angiography results. Ann Thorac Surg. 2009;88:31–9. doi: 10.1016/j.athoracsur.2009.03.081. [DOI] [PubMed] [Google Scholar]
- 21.Kurlansky PA, Traad EA, Dorman MJ, Galbut DL, Zucker M, Ebra G. Thirty-year followup defines survival benefit for second internal mammary artery in propensity-matched groups. Ann Thorac Surg. 2010;90:101–8. doi: 10.1016/j.athoracsur.2010.04.006. [DOI] [PubMed] [Google Scholar]
- 22.Tranbaugh RF, Dimitrova KR, Friedmann P, et al. Radial artery conduits improve long-term survival after coronary artery bypass grafting. Ann Thorac Surg. 2010;90:1165–72. doi: 10.1016/j.athoracsur.2010.05.038. [DOI] [PubMed] [Google Scholar]
- 23.Zacharias A, Schwann TA, Riordan CJ, et al. Late outcomes after radial artery versus saphenous vein grafting during reoperative coronary artery bypass surgery. J Thorac Cardiovasc Surg. 2010;139:1511–8.e4. doi: 10.1016/j.jtcvs.2009.07.068. [DOI] [PubMed] [Google Scholar]
- 24.Puskas JD, Sadiq A, Vassiliades TA, Kilgo PD, Lattouf OM. Bilateral internal thoracic artery grafting is associated with significantly improved long-term survival, even among diabetic patients. Ann Thorac Surg. 2012;94:710–5. doi: 10.1016/j.athoracsur.2012.03.082. [DOI] [PubMed] [Google Scholar]
- 25.Habib RH, Schwann TA, Engoren M. Late effects of radial artery versus saphenous vein grafting in patients aged 70 years or older. Ann Thorac Surg. 2012;94:1478–84. doi: 10.1016/j.athoracsur.2012.05.030. [DOI] [PubMed] [Google Scholar]
- 26.Schwann TA, Engoren M, Bonnell M, Clancy C, Habib RH. Comparison of late coronary artery bypass graft survival effects of radial artery versus saphenous vein grafting in male and female patients. Ann Thorac Surg. 2012;94:1485–91. doi: 10.1016/j.athoracsur.2012.05.029. [DOI] [PubMed] [Google Scholar]
- 27.Schwann TA, Al-Shaar L, Engoren M, Habib RH. Late effects of radial artery vs saphenous vein grafting for multivessel coronary bypass surgery in diabetics: a propensity-matched analysis. Eur J Cardiothorac Surg. 2013;44:701–10. doi: 10.1093/ejcts/ezt061. [DOI] [PubMed] [Google Scholar]
- 28.Schwann TA, Tranbaugh RF, Dimitrova KR, et al. Time-varying survival benefit of radial artery versus vein grafting: a multiinstitutional analysis. Ann Thorac Surg. 2014;97:1328–34. doi: 10.1016/j.athoracsur.2013.09.096. [DOI] [PubMed] [Google Scholar]
- 29.Buxton BF, Shi WY, Tatoulis J, Fuller JA, Rosalion A, Hayward PA. Total arterial revascularization with internal thoracic and radial artery grafts in triple-vessel coronary artery disease is associated with improved survival. J Thorac Cardiovasc Surg. 2014;148:1238–43. doi: 10.1016/j.jtcvs.2014.06.056. [DOI] [PubMed] [Google Scholar]
- 30.Austin PC. Using the standardized difference to compare the prevalence of a binary variable between two groups in observational research. Commun Stat Simul Comput. 2009;38:1228–34. [Google Scholar]
- 31.Taggart DP. CABG or stents in coronary artery disease: end of the debate? Lancet. 2013;381:605–7. doi: 10.1016/S0140-6736(13)60258-5. [DOI] [PubMed] [Google Scholar]
- 32.Cohen DJ, Osnabrugge RL, Magnuson EA, et al. for the SYNTAX Trial Investigators Cost-effectiveness of percutaneous coronary intervention with drug-eluting stents versus bypass surgery for patients with 3-vessel or left main coronary artery disease: final results from the Synergy Between Percutaneous Coronary Intervention With TAXUS and Cardiac Surgery (SYNTAX) Trial. Circulation. 2014;130:1146–57. doi: 10.1161/CIRCULATIONAHA.114.009985. [DOI] [PubMed] [Google Scholar]
- 33.Tatoulis J, Buxton BF, Fuller JA. The right internal thoracic artery: the forgotten conduit— 5,766 patients and 991 angiograms. Ann Thorac Surg. 2011;92:9–15. doi: 10.1016/j.athoracsur.2011.03.099. [DOI] [PubMed] [Google Scholar]
- 34.Alexander JH, Hafley G, Harrington RA, et al. for the PREVENT IV Investigators Efficacy and safety of edifoligide, an E2F transcription factor decoy, for prevention of vein graft failure following coronary artery bypass graft surgery: PREVENT IV: a randomized controlled trial. JAMA. 2005;294:2446–54. doi: 10.1001/jama.294.19.2446. [DOI] [PubMed] [Google Scholar]
- 35.Dimitrova KR, Hoffman DM, Geller CM, Dincheva G, Ko W, Tranbaugh RF. Arterial grafts protect the native coronary vessels from atherosclerotic disease progression. Ann Thorac Surg. 2012;94:475–81. doi: 10.1016/j.athoracsur.2012.04.035. [DOI] [PubMed] [Google Scholar]
- 36.Hayward PA, Zhu YY, Nguyen TT, Hare DL, Buxton BF. Should all moderate coronary lesions be grafted during primary coronary bypass surgery? An analysis of progression of native vessel disease during a randomized trial of conduits. J Thorac Cardiovasc Surg. 2013;145:140–8. doi: 10.1016/j.jtcvs.2012.09.050. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


