Figure 4. hMSCs delivered in mECM hydrogel adhere to full-thickness defects in meniscal explants.
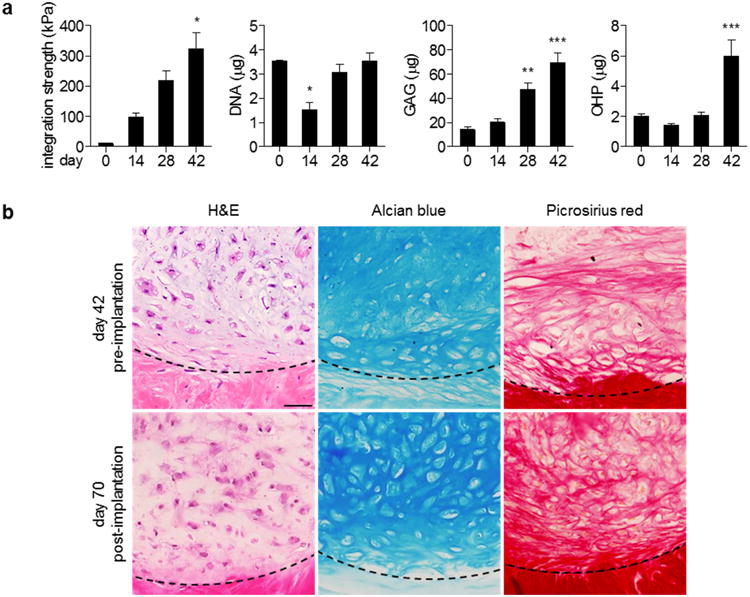
(a) Integration strength and biochemical content of hMSC-mECM explants over six weeks in vitro. The development of integration strength correlated with significant production of sulfated GAG and collagen. Integration strength: * p < 0.05 vs. days 0, 14; n = 3-8. DNA, GAG, OHP: * p < 0.05 vs. days 0, 28, 42; ** p < 0.05 vs. days 0, 14; *** p < 0.05 vs. days 0, 14, 28; n = 3-8. (b) Representative images of explants before and after four weeks of in vivo subcutaneous implantation. Well-developed lacunae appear throughout the sample, whereas those before implantation are confined at the defect interface, indicated by dashed lines. Scale bar: 50 μm.
