Figure 3.
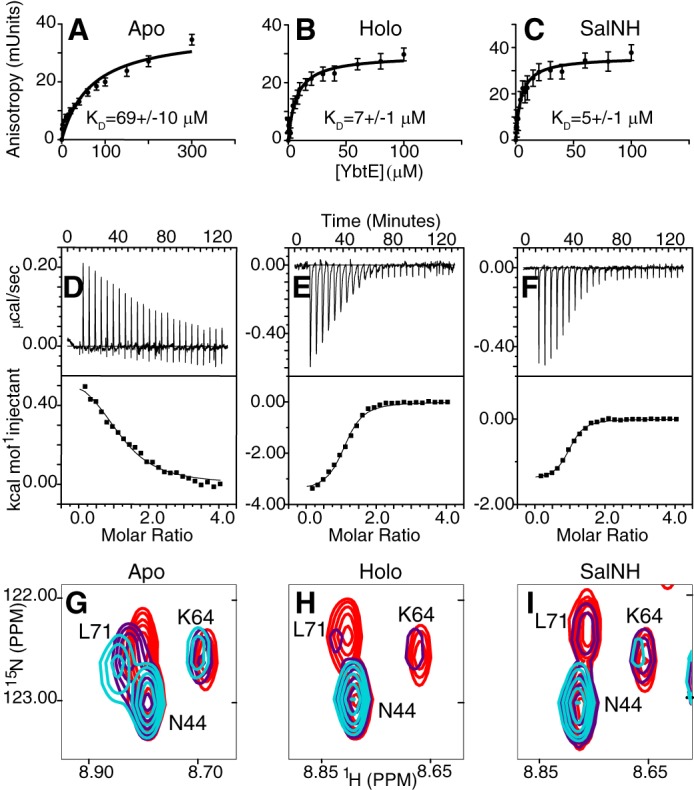
Binding of YbtE and ArCP in apo-form (A, D, and G), holo-form (B, E, and H), and SalNH-loaded form (C, F, and I). A–C, fluorescence anisotropy titrations show that YbtE binds to holo- and SalNH-ArCP with dissociation constants 10-fold lower than that of apo-ArCP. Error bars, S.D. of six (apo-ArCP) or five (holo- and SalNH-ArCP) titrations. The concentration of ArCP was 100 nm. D–F, isothermal titration calorimetry reveals that the interaction with apo-ArCP is driven entirely by entropy and slightly endothermic (D), whereas those of holo- and SalNH-ArCP are exothermic and supplemented with favorable entropy. ArCP was in the cell and at a concentration of 40 μm. The top panels show baseline-corrected raw data, and the bottom panels show the integrated heats. G–I, different forms of ArCP show different spectroscopic responses to binding by YbtE. Select regions of HN-HSQCs of 0.1 mm 15N-ArCP in the presence of YbtE at stoichiometries of 1:0 (red), 1:0.125 (purple), and 1:0.25 (cyan) are shown for apo-ArCP (G), holo-ArCP (H), and SalNH-ArCP (I). All spectra were scaled to identical contour levels.
