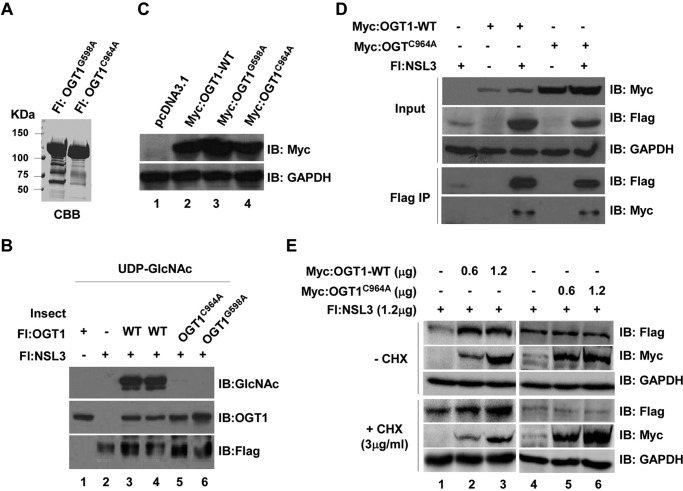
Figure 6.
The O-GlcNAc transferase activity of OGT1 is tightly associated with its stabilization of NSL3. A, purified OGT1mt proteins. Insect cell-expressed and purified point-mutated FLAG-tagged OGT1 proteins (OGT1G598A and OGT1C964A) were visualized by Coomassie Brilliant Blue (CBB) staining. B, loss of O-GlcNAc transferase activity of OGT1mt. An O-GlcNAc transferase assay was performed using OGT1-WT or OGT1mt. Modified NSL3 was detected by Western blotting with anti-GlcNAc antibody. C, validation of OGT1-WT and OGT1mt mammalian expression plasmids. Myc-tagged OGT1-WT and OGT1mt plasmids were transiently transfected into 293T cells. Expressed proteins were checked by Western blotting with anti-Myc antibody. pcDNA3.1 vector is a negative control. D, binding activity of OGT1mt and NSL3. Cells were co-transfected with FLAG-NSL3 and Myc-OGT1-WT/OGTC964A as indicated. 48 h later, FLAG-IP was performed with prepared whole-cell lysates. Bound proteins were analyzed by Western blotting. E, stability of NSL3 protein in OGT1-WT- or OGT1mt-overexpressing 293T cells in the presence or absence of CHX. 293T cells were treated with the translation inhibitor CHX (3 μg/ml) 6 h before harvesting. Western blot analysis was performed after anti-FLAG affinity purification. IB, immunoblot; Fl, FLAG.