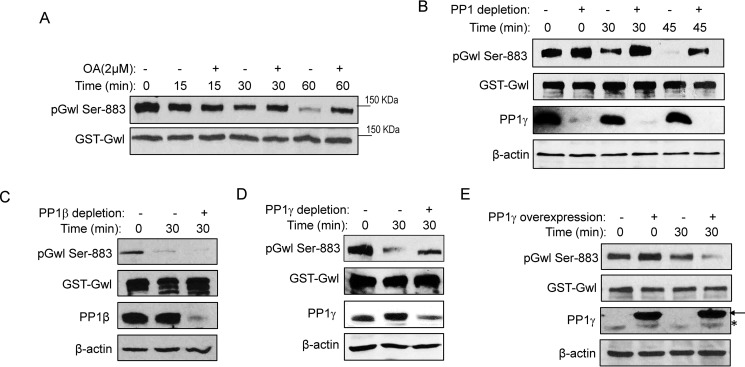
Figure 2.
PP1 mediates Gwl dephosphorylation at Ser-883. A, to prephosphorylate Gwl, GST-Gwl beads were incubated in metaphase-arrested CSF extracts for 30 min and reisolated. GST-Gwl beads were then incubated in interphase egg extracts with or without okadaic acid (OA) for 0, 15, 30, and 60 min. GST-Gwl beads were reisolated and analyzed by immunoblotting for phospho-Gwl Ser-883 and Gwl. B, GST-Gwl was prephosphorylated as in A and then incubated in interphase extracts with or without PP1 depletion for 0, 30, and 45 min. To deplete PP1 from interphase egg extracts, the PP1-binding domain of Pnuts was purified on beads, incubated in extracts for 30 min, and then removed. A mock depletion was performed as a control. Depletion of PP1 was confirmed by immunoblotting for PP1γ and β-actin. Dephosphorylation of Gwl Ser-883 in extracts with or without PP1 depletion was measured by immunoblotting for phospho-Gwl Ser-883 and Gwl. C, GST-Gwl was prephosphorylated as in A and then incubated in interphase extracts with or without PP1β immunodepletion for 30 min. Dephosphorylation of Gwl Ser-883 was measured by immunoblotting for phospho-Gwl Ser-883, Gwl, PP1β, and β-actin. D, GST-Gwl was prephosphorylated as in A and then incubated in interphase extracts with or without PP1γ immunodepletion for 30 min. Dephosphorylation of Gwl Ser-883 was measured by immunoblotting for phospho-Gwl Ser-883, Gwl, PP1γ, and β-actin. E, GST-Gwl was prephosphorylated as in A and then incubated in interphase extracts with or without supplementation of purified His-PP1γ as in Ref. 51. Dephosphorylation of Gwl Ser-883 was measured by immunoblotting for phospho-Gwl Ser-883, Gwl, PP1γ, and β-actin. The arrow points to His-PP1γ, and the asterisk marks the endogenous PP1γ. The data in this figure are representative of three or more independent experiments.