Figure 6.
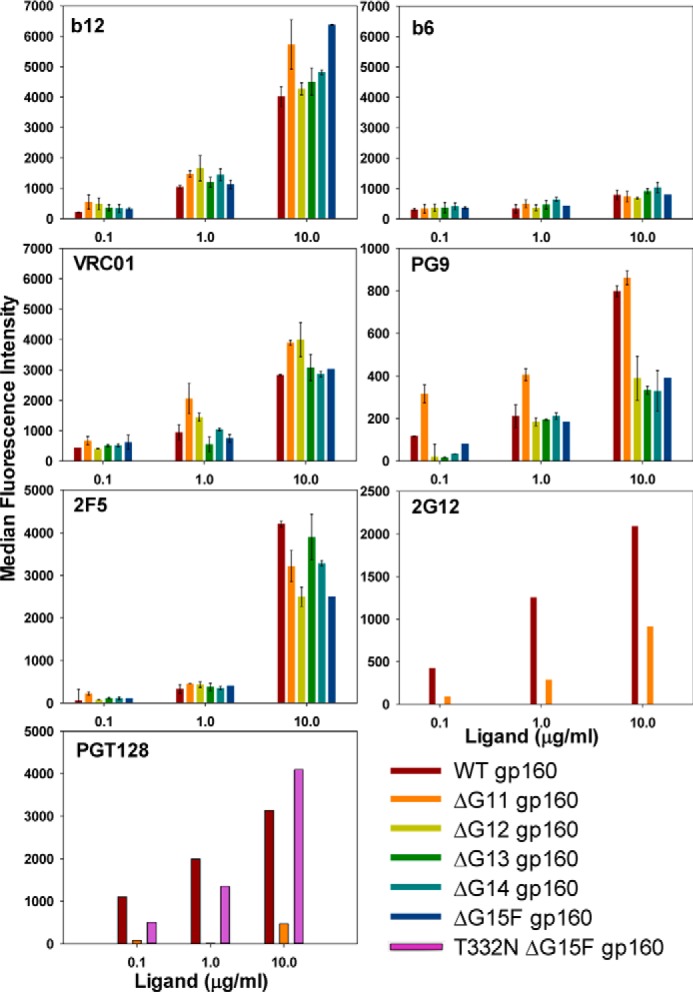
FACS-based mammalian cell-surface staining plots of JRFL cleavage-competent WT gp160 and its glycan derivatives for binding to various anti-HIV-1 antibodies. ΔG11 gp160 (devoid of 11 outer domain glycans) binds to the anti-gp120-neutralizing monoclonal antibodies b12, VRC01, PG9, and anti-gp41 mAb 2F5 similar to the WT gp160, and like the WT it binds poorly to the non-neutralizing mAb b6, confirming that it is properly folded. It does not bind to the neutralizing mAb PGT128 as it lacks the Asn-332 glycan. Other glycan-deficient derivatives of JRFL gp160 also show a binding pattern similar to ΔG11 gp160. Reintroduction of the Asn-332 glycan in ΔG15F gp160 (bottom panel) restores PGT128 binding. Error bars represent standard deviation for the data from two independent experiments.
