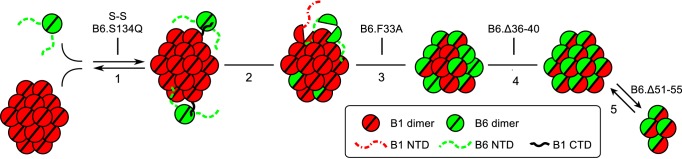
Figure 9.
Schematic summarizing the regions of HSPB6 involved in hetero-oligomer formation. 1) CTD of HSPB1 recruits HSPB6 via patching of the hydrophobic groove formed between the β4- and β8-strands of the ACD (represented by semi-circle) of the latter sHSP. This interaction is blocked by mutation of Ser-134 in HSPB6 and by disulfide cross-linking (S–S) of both proteins (21, 43). 2) Residue Phe-33 of the NTD of HSPB6 destabilizes the NTD interactions in the HSPB1 homo-oligomer permitting subunit exchange and full incorporation of HSPB6. 3) Within the resultant heterocomplex, the individual subunits can freely exchange. 4) Preferential heterodimerization within the mixed oligomer is driven by residues 36–40 of HSPB6. 5) The hetero-oligomer is found in an equilibrium of two species, the populations of which are regulated by residues 51–55 in HSPB6.