Figure 3.
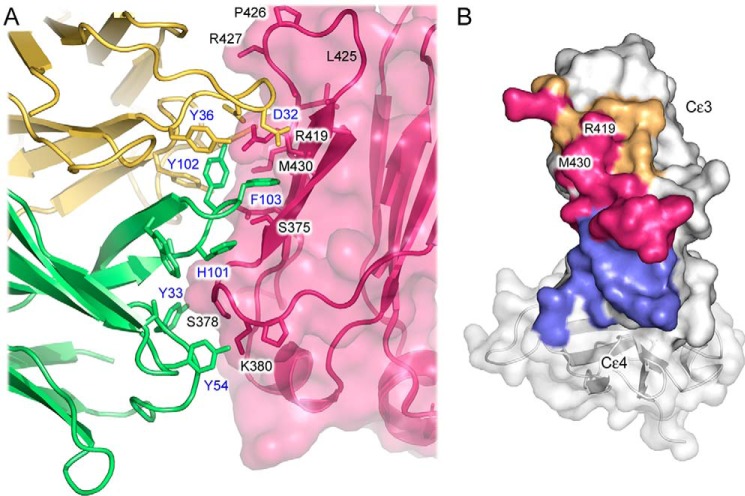
A, interface between FabXol3 and IgE-Fc. The interface between FabXol3 Fab2 (heavy and light chains colored in green and yellow, respectively) and the Cϵ3 domain from IgE-Fc (pink) is shown. FabXol3 and Cϵ3 domain residue labels are colored blue and black, respectively. The interface includes hydrogen bonds and van der Waals interactions. A notable feature of the interface is a cation/π interaction between Arg-419 (Cϵ3 domain) and Phe-103 (FabXol3 CDRH3). The Phe-103 side chain is mostly buried in a pocket created by Thr-373, Trp-374, Ser-375, Gln-417, and Arg-419 (Cϵ3 domain). B, FabXol3 and DARPin E2_79 (21) bind to an overlapping interface on the Cϵ3 domain. IgE-Fc residues, which only form part of the FabXol3 interface, are colored orange, and those that only form part of the DARPin E2_79 interface, which includes part of the Cϵ3-Cϵ4 linker, are colored in blue. IgE-Fc residues colored in pink, which include Arg-419 and Met-430, are common to both FabXol3 and DARPin E2_79 interfaces.