Abstract
Neurologic complications of cancer are common and are frequently life-threatening events. Certain neurologic emergencies occur more frequently in the cancer population, specifically elevated intracranial pressure, epidural cord compression, status epilepticus, ischemic and hemorrhagic stroke, central nervous system infection, and treatment associated neurologic dysfunction. These emergencies require early diagnosis and prompt treatment to ensure the best possible outcome and are best managed in the intensive care unit. This article reviews the presentation, pathophysiology, and management of the most common causes of acute neurologic decompensation in the patient with cancer.
Keywords: Neuro-oncology, elevated intracranial pressure, cord compression, status epilepticus, stroke
Introduction
Cancer is the second leading cause of death in the United States; yet patients with cancer are living longer with the disease. With increasing life expectancy, neurologic complications of cancer are becoming increasingly prevalent.
Neuro-oncology as a specialty, focuses on the treatment of primary malignancies of the central nervous system, and also deals with the neurologic complications of cancer, which occur in approximately 15% of cancer patients over the course of treatment.1 Neurologic complications of primary brain tumors and systemic cancer are serious, and frequently result in hospital admission. In one retrospective review, neurologic complications accounted for 50% of admissions to a solid tumor service at a tertiary care center.2 These complications result in significant morbidity and may be life-threatening emergencies that require urgent intervention, intensive monitoring, respiratory support due to depressed mental status, and management of systemic complications such as pulmonary embolus, myocardial infarction, pneumonia, and sepsis.
The neurologic emergencies that occur as a complication of cancer are diverse and are outlined in Table 1. When left untreated, these conditions result in irreversible injury; hence, early diagnosis and intervention is critical. This article discuses the clinical presentation and both, available and emerging treatment options.
Table 1. Neurologic emergencies and their critical care needs.
| Neurologic Emergency | Critical Care Needs |
|---|---|
Elevated intracranial pressure
|
Frequent neurochecks, osmotherapy, intubation/airway management for depressed mental status, neurosurgical management (ICP monitoring, continuous CSF drainage) |
Status epilepticus
|
Frequent neurochecks, intubation/airway management for depressed mental status, propofol or midalozolam infusions, vEEG monitoring |
| Cord compression | Frequent neurochecks, management of spinal shock |
| Ischemic stroke | Frequent neurochecks, monitoring for hemorrhagic conversion (particularly, after the administration of tPA or following thrombectomy), monitoring for herniation in patients with a malignant MCA distribution stroke. |
| Intraparenchymal brain hemorrhage | Frequent neurochecks, intensive blood pressure control, reversal of coagulopathy/thrombocytopenia, intubation/airway management for depressed mental status, management of complications such as herniation and status epilepticus. |
| Venous sinus thrombosis | Frequent neurochecks, management of complications like intraparenchymal brain hemorrhage and seizures. |
| Meningoencephalitis | Frequent neurochecks, intubation/airway management for depressed mental status, management of complications such as elevated intracranial pressure and seizures |
| Neurologic complications of treatment | Frequent neurochecks, intubation/airway management for depressed mental status, management of seizures and associated medical complication. |
Elevated Intracranial Pressure
In patients with cancer, a frequent neurologic emergency is the development of elevated intracranial pressure (ICP). When it is not appropriately managed, elevated ICP rapidly results in irreversible neurologic deficits and death.
The pathophysiology of increased ICP can be understood by the Monro-Kellie Doctrine—a cornerstone of neurologic intensive care. The volume of the cranial vault ranges from 1400 to 1700 cc, of which the brain constitutes approximately 80% of that volume, and CSF and blood, each constitute another 10%.3 Since the cranium is rigid and has a fixed volume, the Monro-Kellie Doctrine states that an increase in the volume occupied by brain, CSF, or blood, results in a decrease in one or both of the other components, or the system's ability to compensate fails giving rise to an increase in ICP.4
Cerebral perfusion pressure is equal to the difference between the mean arterial pressure and intracranial pressure; hence, elevated ICP if left untreated results in cerebral ischemia.5 A rise in ICP also places patients at risk for herniation since it leads to the development of a pressure gradient, which the brain attempts to normalize by shifting the contents of one compartment into the adjacent, lower pressure, compartment.6
There are many etiologies of increased ICP in patients with cancer. Primary and metastatic brain tumors exert mass effect because the neoplasm occupies space, but also because the tumor increases vascular permeability causing vasogenic edema. Tumors can also increase intracranial pressure during the first 3 to 6 months after radiotherapy, as a consequence of a robust inflammatory reaction that disrupts the blood brain barrier and results in new areas of contrast enhancement and edema. This complication of radiotherapy is known as pseudoprogression and is virtually indistinguishable from tumor progression. Pseudoprogression may be differentiated from true tumor progression by observing the enhancing abnormality over time—in pseudoprogression, the enhancement should stabilize or spontaneously decrease.7 A similar treatment effect that results in a mass-like area of contrast enhancement that mimics tumor progression, occurs from 6 months to 2 years after radiation and is known as radiation necrosis (shown in figure 1). Radiation necrosis can occur following fractionated radiotherapy or after stereotactic radiosurgery and is thought to be due to vascular damage, which induces tissue hypoxia. Similar to pseudoprogression, it causes disruption of the blood-brain barrier, which may result in significant vasogenic edema.8 As the name implies, biopsy of an enhancing abnormality caused by radiation necrosis, reveals bland necrosis rather than viable tumor cells (figure 2). Differentiating between tumor and treatment effects is an important diagnostic dilemma in neuro-oncology. It may be aided by advanced imaging techniques such as FDG-PET and MRI perfusion imaging (figure 1D); however, these imaging modalities have not been studied prospectively and are not standard of care.9
Figure 1.
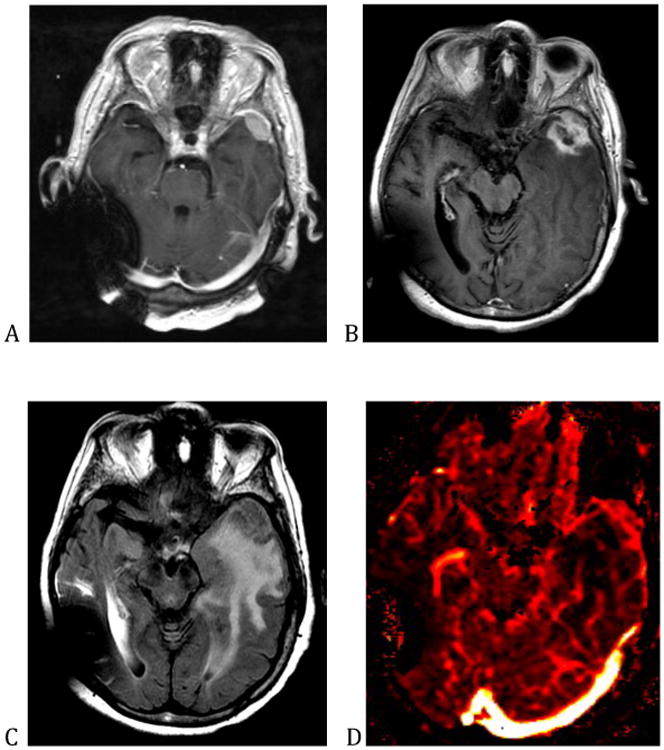
This is a patient with a meningioma. (A) shows the appearance of the meningioma on the T1 post-contrast sequence prior to stereotactic radiosurgery. (B) shows the enlargement of the contrast enhancing abnormality in the immediate period after stereotactic radiosurgery, as a consequence of pseudoprogression. (C) shows the associated vasogenic edema. (D) shows that the lesion has decreased cerebral blood volume on perfusion imaging, suggesting that the increase in contrast enhancement is due to treatment effect rather than tumor growth.
Figure 2.
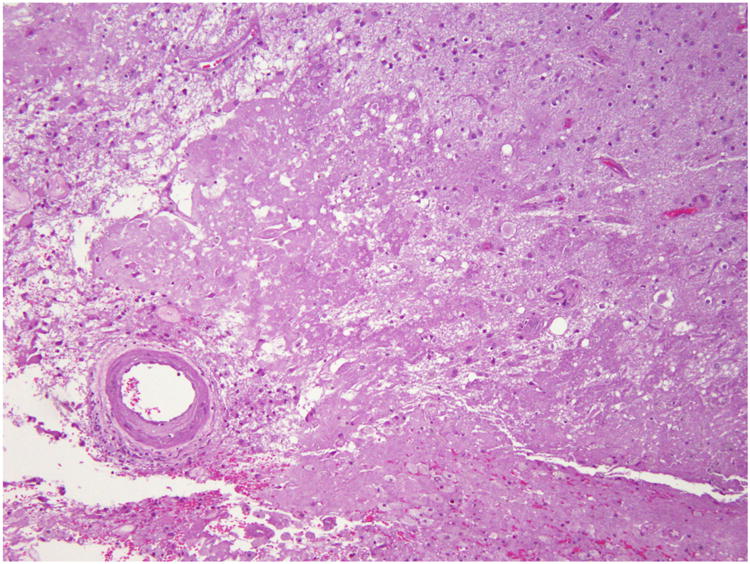
The histological appearance of radiation necrosis. The radiation necrosis is characterized by large zones of necrosis that primarily involves the white matter, and fibrinoid necrosis or hyalinization of blood vessels.
Increased ICP may also occur as a consequence of cytotoxic edema, which is an accumulation of fluid in the intracellular space that occurs due to status epilepticus, meningoencephalitis, hyperammonemia, and stroke. Another cause of increased ICP is decreased CSF outflow, which is seen in patients with an obstruction of the ventricular system and in patients with decreased CSF absorption. Finally, hemorrhage into the cranial vault will also create mass effect and also increases intracranial pressure.
Headache is the most common symptom of elevated intracranial pressure. The traditional teaching is that the headache that occurs in the setting of elevated ICP is more prominent in the morning as a result of nocturnal hypercarbia, which causes vasodilation, and supine posture. However, Forsyth and Posner found that the classic morning headache is actually uncommon and only present in 17% of patients.10 Another characteristic of the headache that is attributable to ICP is plateau wave phenomena. Plateau waves are sudden increases in ICP from the normal range (< 15 mmHg) to 50-100 mmHg;11 they are transient, last for 5-20 minutes, and can occur spontaneously or can be precipitated by coughing, pain, and changes in position from lying to sitting or sitting to standing.12 With this sudden elevation in intracranial pressure, individuals not only develop headaches but also transient neurologic deficits, such as depressed arousal or focal deficits. Hence, plateau waves are frequently misattributed to orthostasis.
Other symptoms of increased ICP include nausea, vomiting, and diplopia from abducens (CN VI) nerve palsies. The abducens nerve follows a long tortuous course from the pons to the lateral rectus; increased intracranial pressure causes downward displacement of the brainstem resulting in dysfunction of the nerve as it is stretched.13 When it occurs, the abducens nerve palsy falsely localizes to the pontomedullary junction, as the true etiology of the deficit is a mass causing increased ICP that resides elsewhere. Besides deforming the brainstem, elevated intracranial pressure is transmitted to the optic nerve, giving rise to optic disc swelling (papilledema) and loss of venous pulsations. For this reason, an ophthalmologic exam is a crucial component of the evaluation of a patient suspected of having high ICP.
An end-stage manifestation of elevated intracranial pressure is the Cushing's reflex, which is caused by brainstem compression, brainstem stretch or a combination of both. The Cushing's reflex is the triad of hypertension, bradycardia, and irregular breathing; it suggests developing cerebral ischemia, imminent herniation, and the need to act promptly.14,15
Brain Herniation
Brain herniation occurs when a pressure gradient causes cerebral structures to enter adjacent compartments (figure 3). Uncal herniation develops when a hemispheric lesion pushes the uncus of the temporal lobe (the hippocampal gyrus) into the posterior fossa, resulting in compression of the midbrain and the ipsilateral oculomotor nerve (CN III).16 A third nerve palsy causes: (1) the affected eye to be depressed and abducted (“down and out”), (2) ipsilateral ptosis from denervation of the levator palpebrae superioris, and (3) mydriasis from unopposed sympathetic activation, resulting in a dilated pupil, also called a “blown pupil.” A “blown pupil” may occur prior to the oculomotor deficits of a third nerve palsy, since the parasympathetic fibers run along the outside of the nerve and are therefore, the first to be affected. Uncal herniation may compress the posterior communicating arteries as they pass the tentorium, resulting in occipital lobe strokes. It may also cause hemiparesis, which is ipsilateral to the supratentorial lesion that is causing the uncal herniation. This is known as Kernohan's notch phenomenon, and it occurs as a result of displacement of the midbrain by the uncus and compression of the contralateral cerebral peduncle against the tentorium cerebelli.17,18
Figure 3.
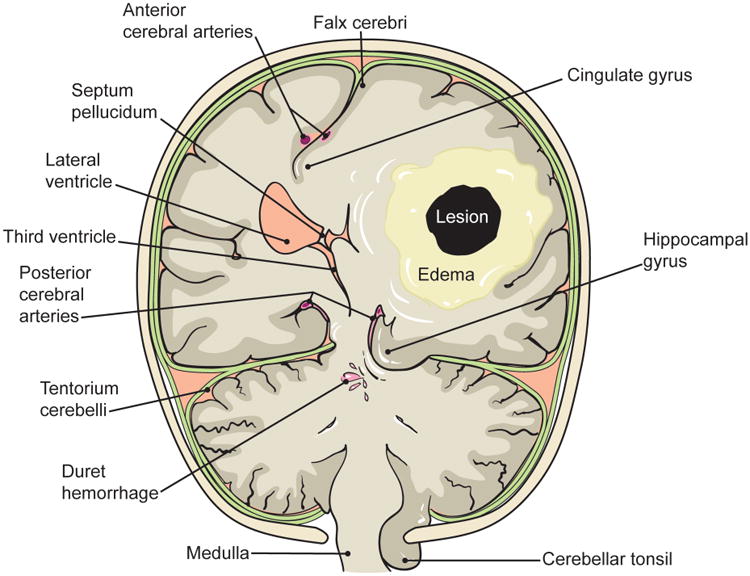
A large supratentorial lesion may cause uncal herniation, where the hippocampal gyrus is forced into the posterior fossa, resulting in compression of the midbrain and the ipsilateral third nerve. Uncal herniation may also cause Kernohan's notch phenomenon, where the contralateral corticospinal tract becomes compressed against the tentorium cerebelli as it passes through the midbrain. A large supratentorial mass may also result in midline shift and subfalcine herniation, which may cause a stroke in the ipsilateral anterior cerebral artery distribution. Finally, downward herniation can cause a pontine hemorrhage and herniation of the cerebellar tonsils, resulting in dysfunction of the medulla.
The brain can herniate downward onto the brainstem. When this occurs the reticular activating system and other vital brainstem functions may be affected, either through direct compression or compression and stretching of the small penetrating arteries of the pons leading to a catastrophic hemorrhage.19 A hemorrhage that is isolated to the ventral pons, which spares the reticular activating system, causes a devastating neurologic syndrome, known as the locked-in syndrome. It manifests as quadriplegia and cranial nerve paralysis with preserved consciousness. In classic locked-in syndrome, first described in 1966 by Plum and Posner, the only volitional movements that remain are upgaze and upper eyelid movements.20,21
Depending on the location and size, a supratentorial lesion may also cause the cingulate gyrus to herniate under the falx cerebri. This is known as subfalcine herniation and it results in compression and infarction of the territory supplied by the ipsilateral anterior communicating artery.17 Clinically, patients experience contralateral leg weakness and bladder incontinence when there is dysfunction of the bilateral medial frontal micturition centers.22 Additionally, large infratentorial (posterior fossa) mass lesions may result in upward herniation of the vermis into the brainstem, dorsal compression of the pons, and downward herniation of the cerebellar tonsils into the foramen magnum; neurologic deficits may be further compounded by the development of hydrocephalus (figure 4).23
Figure 4.
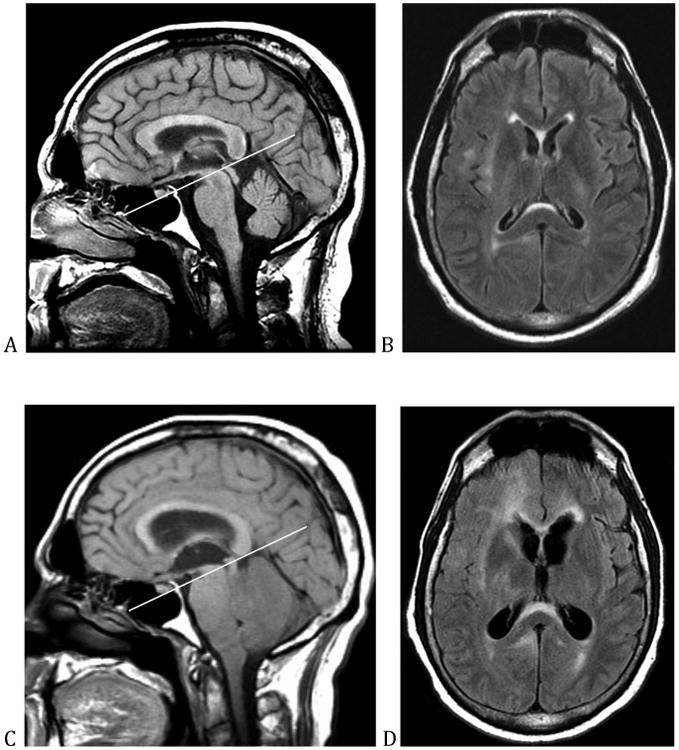
This is a patient with an anaplastic astrocytoma. (A) and (B) show the appearance of the patient's brain at presentation. (C) shows the effect of neoplastic infiltration on the patient's cerebellum, which results in compression of the brainstem, upward and downward herniation, and closure of the fourth ventricle. (D) shows the resultant hydrocephalus.
Patients with elevated ICP, who are developing herniation, are frequently stuporous and so the management of these patients begins with securing the airway. In a patient with elevated intracranial pressure, sedation lowers intracranial pressure at the expense of the neurologic exam, which is critical in the management of these patients. Sedation in these patients is therefore a fine balance between controlling ICP and keeping the patient sufficiently awake so that frequent neurochecks can be performed to evaluate for further neurologic deterioration.
Dexamethasone, a corticosteroid with minimal mineralocorticoid activity, is effective at reducing vasogenic edema regardless of the cause (tumor or treatment effect); hence, it is the first line treatment in a patient with a mass lesion who presents with elevated ICP. In patients with a mass lesion with significant vasogenic edema, a typical regimen is a loading dose of dexamethasone 10 to 20 mg followed by a maintenance dose of 8 to 16 mg per day.24 While the total daily dose is frequently divided into four doses, given every 6 hours, because of its long half life, it is also appropriate to dose dexamethasone every 12 hours.1 Unlike patients with vasogenic edema, steroids play no role in the management of patients with herniation due to cytotoxic edema.
In combination with steroids, osmotherapy is the main medical treatment for elevated intracranial pressure. Osmotherapy is typically reserved until the time of herniation as a stopgap measure that bridges patients until the elevated intracranial pressure resolves or until it can be treated definitively. It can be used to rapidly reverse active herniation due to vasogenic edema, in combination with steroids, and is often used to treat elevated ICP due to cytotoxic edema though its effectiveness is less clear in this setting. Hypertonic saline and mannitol are the two agents that are used to create an osmotic gradient across the blood brain barrier to shift fluid out of the brain. These two agents each have their merits and are used interchangeably, though in transtentorial herniation, there is some evidence that 23.4% hypertonic saline is particularly effective.16
Osmotherapy only serves as a stopgap as most processes that cause imminent herniation fail to spontaneously resolve before osmotherapy loses its effectiveness. Neither mannitol nor hypertonic saline can be continued indefinitely as the brain develops idiogenic osmoles which draws water back into the brain, and these agents cause dose limiting metabolic derangements and other toxicities.25
The only other non-surgical interventions are head elevation and hyperventilation to a pCO2 of 26-30 mm Hg.5 These interventions are primarily useful in the hyperacute setting, prior to the initiation of osmotherapy. Hyperventilation decreases ICP by causing vasoconstriction and decreasing cerebral blood volume. Hence, there is a theoretical risk of ischemia with prolonged hyperventilation, which may account for the worse outcome identified by one study that looked at patients with traumatic brain injury who were maintained on hyperventilation.26
In terms of definitive therapy, only selected patients are suitable neurosurgical candidates. Not all patients with a primary or secondary malignancy of the brain will benefit from surgery due to the burden of disease or location of the lesion. Moreover, only certain masses should be resected—for instance, masses due to lymphoma and tumefactive MS should be managed medically. Lymphoma is exquisitely sensitive to steroids, chemotherapy and radiation, and tumefactive MS responds to immunomodulation. Pseudoprogression and radiation necrosis are also managed medically with the exception of particularly robust and refractory cases, which are infrequently surgically resected.27 As previously discussed, it can be difficult to distinguish between pseudoprogression and true tumor progression; likewise, it can be challenging to distinguish between a solid tumor that would benefit from resection, and lymphoma or tumefactive multiple sclerosis. Lymphoma typically gives rise to homogenously enhancing, supratentorial lesions that are frequently subependymal, but can occur in the deep gray matter.28 Tumefactive multiple sclerosis typically produces enhancing lesions that form an incomplete ring and have less edema than mass lesions.28 These imaging characteristics are non-specific and a brain biopsy is often needed to make a definitive diagnosis.
In selected patients with large contrast enhancing tumors with significant vasogenic edema, who are appropriate candidates for further treatment, bevacizumab may temper early herniation. Unlike steroids, which may reduce vasogenic edema within hours and has a maximal effect at 3-6 days, bevacizumab improves edema over days to weeks and is therefore, of little value in the acute management of a patient that is actively herniating. Bevacizumab is a monoclonal antibody that inhibits angiogenesis by blocking the activity of vascular endothelial growth factor (VEGF). Due to its profound effects on vasculature, it is effective at reducing edema and counteracting the inflammatory effects of pseudoprogression and the vasculopathy of radiation necrosis.29-32 It may permit some patients with large contrast enhancing lesions and significant edema, who have been stabilized on steroids, to be weaned off the steroids, and for this reason, it can be considered a steroid-sparing agent.24,33
Hydrocephalus
Hydrocephalus is a common complication of metastatic cancer. There are two types, communicating and non-communicating hydrocephalus.
Communicating hydrocephalus is due to impaired CSF absorption, and in the cancer population, the most common cause is leptomeningeal carcinomatosis. Patients with leptomeningeal carcinomatosis develop hydrocephalus and then present with signs and symptoms of increased ICP. When the process is sufficiently indolent, the ventricles may enlarge without increasing the opening pressure.34 This latter group develops symptoms of normal pressure hydrocephalus, i.e. apraxic gait, incontinence, and progressive confusion.1 Other signs and symptoms of leptomeningeal carcinomatosis that co-occur with hydrocephalus, which help to make the diagnosis, include cranial nerve palsies, radiculopathy, nuchal rigidity, and cortical deficits.35,36
The evaluation of communicating hydrocephalus includes imaging of the entire neuroaxis to look for radiographic evidence of leptomeningeal disease and a lumbar puncture. The purpose of the lumbar puncture is two fold: to sample the CSF for malignancy and to determine whether decompression of the ventricular system palliates the symptoms of hydrocephalus. While an improvement in neurologic symptoms following a high volume CSF drainage suggests that the patient will benefit from a ventriculoperitoneal shunt, patients with large ventricles, with the right clinical phenotype, may benefit from a ventriculoperitoneal shunt in spite of a negative tap test.34
Placing a ventriculoperitoneal shunt in patients with leptomeningeal disease may be followed by radiation to symptomatic areas, treatment with systemic chemotherapy with good blood-brain barrier penetration, and potentially intrathecal chemotherapy in patients who do not have bulky leptomeningeal disease and without significant hydrocephalus.35 Intrathecal chemotherapy may not be appropriate in a patient with overt hydrocephalus because it is a risk factor for the development of a necrotizing leukoencephalopathy.37
Following shunt placement, patients may again develop signs and symptoms of hydrocephalus; when this occurs, consideration should include overdrainage causing subdural hematomas, infection, and shunt malfunction. Shunt failure can be difficult to diagnose, and rapidly fatal if the problem is not corrected. It can occur because of discontinuities or kinks in the tubing, and because shunts become obstructed, particularly when there is an increase in proteinaceous material in the CSF due to leptomeningeal disease. If the shunt was placed to relieve elevated intracranial pressure from communicating hydrocephalus, a lumbar puncture can be helpful in diagnosing a malfunction as the opening pressure of a lumbar puncture should match the pressure setting of a properly functioning shunt.
Non-communicating or obstructive hydrocephalus occurs when a structural lesion obstructs the ventricular system and is a cause of rapid neurologic deterioration. An intraventricular tumor, such as a pineal region tumor will block the third ventricle resulting in dilatation of the bilateral lateral ventricles and downward herniation; alternatively, a hemispheric lesion can entrap a lateral ventricle, resulting in dilatation of that ventricle and uncal herniation. Another catastrophic event is when the fourth ventricle is closed off by an enlarging posterior fossa mass; this results in the situation shown in figure 4C and D.
In patients with non-communicating hydrocephalus, a lumbar puncture would not relieve the accumulation of CSF and is potentially unsafe as it could result in herniation and death. Signs that a lumbar puncture is unsafe are the presence of a lateral shift of midline structures and loss of the suprachiasmatic and perimesencephalic cisterns. A large posterior fossa mass is also a relative contraindication to lumbar puncture.6 For this reason, there is no intermediate step in the management of non-communicating hydrocephalus prior to diversion of fluid via VP shunt or third ventriculostomy, or definitive treatment of the obstructing lesion.
Cord Compression
Spinal cord compression from an epidural metastasis is a common neurologic complication of cancer (occurring in 5-10% of patients). It represents a neuro-oncological emergency because favorable neurologic outcome is contingent on early recognition and management. Cord compression in solid tumors most commonly arises from epidural extension of a vertebral metastasis (∼75% of cases), though vertebral collapse from tumor infiltration may compress the cord (∼20% of cases) and rarely intrathecal deposits may develop.38 Epidural spinal cord compression most frequently involves the thoracic spinal cord (∼70% of cases), but can occur anywhere along the length of the cord, down to L1 where the cord terminates at the conus medullaris.39,40 Beyond L1, high-grade epidural disease causes a cauda equina syndrome due to compression of the nerve roots as they travel past the conus medullaris and is managed similarly.
Because of its high prevalence and the close proximity of the lungs to the vertebral column permitting local extension, lung cancer is the most common etiology; however, epidural spinal cord compression also occurs commonly in breast, prostate, renal cell, and colorectal cancer.39 Hematologic malignancies such as non-Hodgkin lymphoma also cause epidural spinal cord compression, occasionally as the first presentation, but often without bony involvement.41
Since spinal cord compression primarily develops from vertebral metastases, the presenting symptom is commonly bony pain at the site of the deposit. Bony pain from a vertebral metastasis, also known as biologic pain, can be distinguished from arthritic pain because it classically awakens the patient from sleep as endogenous steroid production nadirs during the night, unlike mechanical pain from arthritic changes which is typically exacerbated by activity.39
As vertebral metastases enlarge, in addition to extending dorsally, they can also extend laterally, compressing nerve roots as they are exiting through the neural foramina, causing radicular pain. Radicular pain follows a dermatomal pattern; hence, radicular pain in the trunk causes a band like pain in contrast to radicular pain from a lumbosacral metastasis, which causes an electrical pain that typically radiates down the leg. Another source of discomfort is Lhermitte's sign, which is an electrical sensation that runs down the spine to the arms and the leg that occurs following neck flexion. This phenomenon is due to irritation or compression of the dorsal columns, typically at the cervical spine but also at the upper portion of the thoracic spine.42
Lhermitte's sign suggests cord dysfunction as does weakness, loss of sensation below the level of the lesion, and bladder or bowel dysfunction. These findings indicate that cord compression has begun and urgent intervention is necessary. A lesion above T1 affecting the corticospinal tract or the anterior horn of the spinal cord will cause upper and lower extremity weakness that is typically bilateral though it may be asymmetric. Any lesion affecting those same structures below T1 will only cause lower extremity weakness. A sensory level suggests a localization within the spinal cord, but can be unreliable. The cord compression may be above that level and there may be other sites of disease, and for that reason a MRI of the total spine is indicated.40 While a CT of the spine may identify a culprit lesion, MRI of the spinal cord provides significantly more information, such as cord edema and ischemia, which is important in management. At the time of presentation, patients may exhibit spinal shock resulting in decreased tone and hyporeflexia; over time, compression of the spinal cord, specifically of the corticospinal tracts, gives rise to upper motor neuron signs, such as increased tone, spasticity, and pathologic reflexes—for example, presence of the Babinski sign (extensor toes).
When cord compression occurs at the high cervical cord, a relatively rare occurrence, respiratory compromise results. Both cervical and high thoracic spinal cord compression can cause autonomic dysreflexia, which manifests as bradycardia, extreme hypertension,43 strokes and myocardial infarction. Autonomic dysreflexia can be triggered by constipation or an excessively full bladder, and so these triggers should be recognized and addressed preemptively.
At the time of recognition, treatment should be initiated with a bolus of dexamethasone. The appropriate dose of dexamethasone has been long debated and has not been settled. The typical range that is administered varies from 10-100 mg. One small study enrolled 37 patients to dexamethasone boluses of 10 or 100 mg, and in their small population, they found no difference in outcome in patients that received the lower dose in terms of pain, ambulation or bladder function; however, they did identify more side effects in the high-dose group.44 While this suggests that most patients should receive a dexamethasone bolus of 10 to 16 mg at the time of presentation with cord compression, it may still be reasonable to treat highly symptomatic patients with dexamethasone 100 mg. Following the initial bolus, dexamethasone 4 mg every 6 hours or 8 mg every 12 hours are the typical maintenance dosing schedules.
The NOMS criteria was developed at Memorial Sloan Kettering Cancer Center (MSKCC) to provide a decision making framework for the management of metastatic spine disease. NOMS is an acronym and pneumonic device, which allows the evaluator to remember the four assessments by which spinal metastases should be evaluated: Neurologic, Oncologic, Mechanical instability, and Systemic disease. The neurologic assessment is based on the clinical presentation and the radiologic features of the epidural disease. In a randomized clinical trial, patients with radiologically proven cord compression, neurologic symptoms, and less than 48 hours of paraplegia, were more likely to retain the ability to walk and had improved pain control when treated with surgery followed by radiation compared to radiation alone.45 Based on this trial, high-grade metastatic epidural spinal cord compression is an indication for surgical decompression in patients with disease restricted to a single area and an expected life expectancy of at least 3 months. It is now known that surgery can be avoided in patients with highly radiosensitive/chemosensitive tumors causing high-grade epidural spinal cord compression, and for this reason, an oncologic assessment is required. Tumors that are commonly considered radiosensitive are lymphoma, seminoma, myeloma, breast, prostate, ovarian and neuroendocrine carcinomas. In these tumors, external beam radiation therapy can frequently reverse neurologic deficits. Radioresistant tumors include renal, thyroid, hepatocellular, colon, non-small cell lung carcinomas, sarcoma, and melanoma; however, even these metastases can be treated with highly conformal image-guided external beam radiotherapy in the absence of high-grade epidural spinal cord compression. Mechanical instability is another indication for surgical stabilization by consensus opinion.46 The final assessment in deciding the most appropriate treatment is an evaluation of the extent of systemic disease, as a patient with widely metastatic disease may not be a good candidate for an aggressive surgery.
Whether or not patients receive surgery, palliative radiation is indicated in individuals with epidural spinal cord compression with controlled systemic disease. The dose administered is a tradeoff between tumor control and neurotoxicity to the adjacent spinal cord. Early transient radiation-induced myelopathy occurs 2-6 months after treatment and is thought to occur due to demyelination of the dorsal columns, hence it is associated with Lhermitte's sign; in these patients, the MRI is usually unrevealing.39 Late radiation myelopathy occurs over a year after RT, and is sometimes (though not always) associated with MRI changes and frequently results in painless, progressive paraparesis, sensory loss, and loss of bladder and bowel function and so it is on the differential when patients represent with lower extremity weakness.39
Status epilepticus
Seizures are highly prevalent in patients with primary and secondary brain tumors. Patients with brain tumors frequently develop symptomatic epilepsy due to cortical irritability resulting from the structural abnormality. Nevertheless, a subset of patients with brain tumors never have a seizure and so prophylaxis with anti-epileptic drugs is not recommended.47 When seizures occur, they start as partial seizures that may or may not secondarily generalize. Low-grade gliomas are more epileptogenic than glioblastoma with an incidence of 60-85%, compared to 30-60% in the latter group.48 In patients with brain metastases, the incidence ranges from 20-35%.49
Most seizures are short and self-limited. They become a neurologic emergency when the seizure evolves into status epilepticus. It has been suggested that cortical injury occurs after 20 to 30 minutes of seizure activity. Using that as a benchmark, it has been suggested that status epilepticus should be defined as a single continuous seizure that exceeds anywhere from 5 to 30 minutes.50 In practicality, patients should be treated as though they are in status epilepticus when a seizure lasts longer than 5 minutes or two or more seizures occur without a return to baseline in between events.
Seizures can be non-convulsive. In this situation, status epilepticus may be a challenging diagnosis to make, as the clinical manifestations are subtle, by definition. For this reason, an EEG is often indicated in the evaluation of a patient that does not return to baseline after a seizure and in the evaluation of altered mental status. Non-convulsive status epilepticus frequently starts with a convulsive seizure, though this is not always the case. Following the initial convulsion, patients may appear to be in a post-ictal state, but in actuality, they are continuing to have seizures.50 On closer inspection, these patients may demonstrate subtle signs of further seizure activity such as eye deviation away from the seizure focus or subtle motor manifestation, such as face or hand twitching.
Seizures occur in patients with cancer: (1) as a neurologic complication of treatment, (2) as a complication of metabolic derangement caused by cancer, (3) due to paraneoplastic limbic encephalitis, and (4) as a result of meningoencephalitis.
Posterior reversible encephalopathy syndrome (PRES) is a common treatment related cause of seizures. In addition to seizure, PRES causes altered mental status, headache, and cortical blindness. It is associated with the following therapies: bevacizumab, sorafenib, cyclophosphamide, l-asparaginase, cisplatin, and gemcitabine; immunosuppressants such as tacrolimus and high-dose corticosteroids; and occurs as a consequence of uncontrolled hypertension resulting in auto-regulatory failure.51,52 Seizures may be triggered by medications that lower the seizure threshold, such as the antibiotics: meropenem, imipenem, cefepime, and ciprofloxacin; pain medications such as tramadol; and anti-depressants like bupropion.
The electrolyte abnormalities that place cancer patients at risk for seizure are the same as for the general population. They are hyponatremia, hypernatremia, hypocalcemia, and hypomagnesemia. These electrolyte abnormalities typically cause generalized tonic-clonic seizures, although partial seizures can also occur.53 Paraneoplastic antibodies that cause seizures by inducing a limbic encephalitis, include anti-Hu, anti-voltage gated potassium channels, anti-GABA receptor, and anti-NMDA receptor antibodies. Removal of these auto-antibodies with IVIG and plasmapheresis can potentially treat the encephalitis.54 In addition to seizures, limbic encephalitis manifests with altered mental status, and on MRI, it frequently results in T2 hyperintensity of the bilateral temporal lobes.
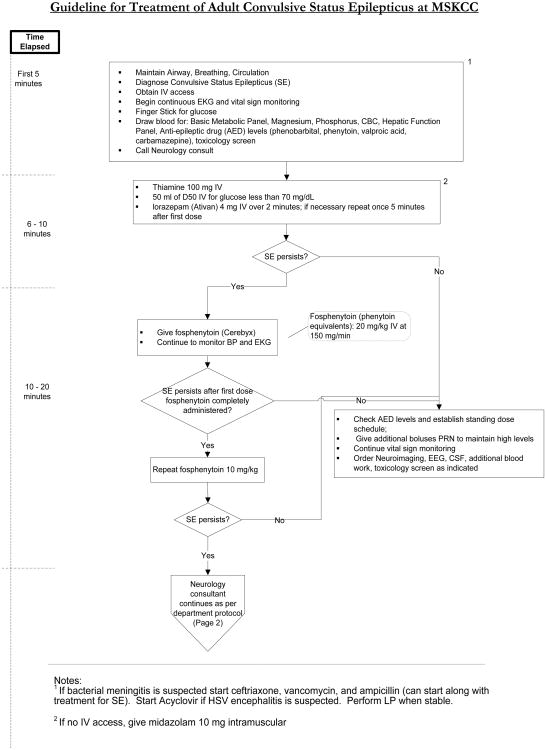
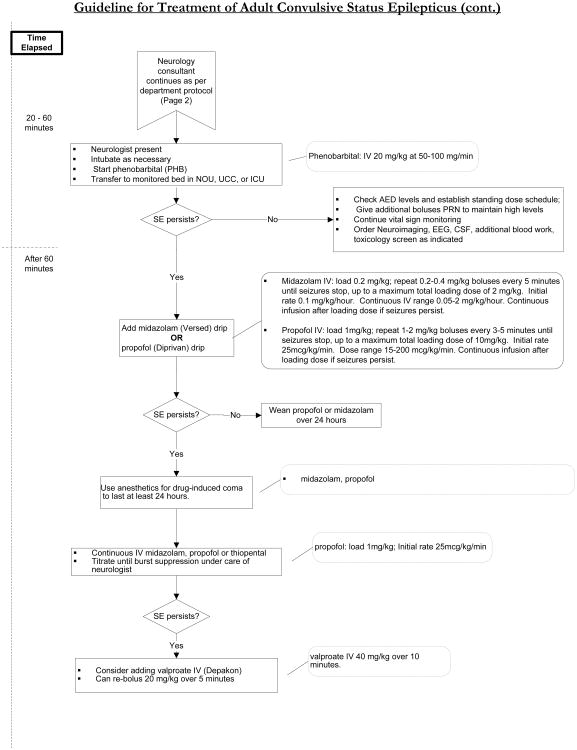
The management algorithm at MSKCC (figure 5) for a patient that presents with a convulsive seizure that lasts longer than five minutes begins with lorazepam 4 mg IV push, since benzodiazepines are the most effective drug at terminating status epilepticus.55 If the seizure does not terminate after 5 minutes, then another 4 mg of lorazepam should be administered. In a patient in whom IV access cannot be obtained, IM midazolam is an option as it appears to be as effective for controlling seizures, with a safety profile that is similar to IV lorazepam when studied prospectively in the prehospital setting.56
Figure 5.
Treatment algorithm for adult convulsive status epilepticus at MSKCC.
For decades, phenytoin has been the anti-epileptic drug of choice for treating status epilepticus. While there are no randomized trials establishing its superiority over other anti-epileptic drugs, phenytoin has the most literature supporting its effectiveness. For that reason, it is still the most frequently used second line agent after benzodiazepines have failed.57 It is loaded at a dose of 15-20 mg/kg with a goal level of 20 mcg/ml (after correcting for hypoalbuminemia). When phenytoin is given in its IV form, it should be infused slowly (no faster than 50 mg/min) because it has the potential to cause hypotension and serious skin reactions. Fosphenytoin is a water-soluble phosphate ester prodrug that is converted to phenytoin within the body.58 It can be infused rapidly with fewer complications than phenytoin; however, hypotension and arrhythmia can still occur with fast infusions at high doses, so patients should be on telemetry and vitals should be checked frequently during administration and for at least twenty minute afterwards.
If phenytoin fails to control the seizure, several medications can be considered as third-line agents: specifically phenobarbital, valproic acid, lacosamide, and levetiracetam.59 Frequently, patients in status epilepticus have lost their ability to protect their airway, are hypercarbic prior to consideration of a third line agent, and require intubation. Patients that are intubated can be placed on propofol or midazolam infusions, which is very effective at suppressing seizures. The rate should be titrated to the absence of seizure on video EEG and a burst suppression pattern in refractory cases.
In non-convulsive status, the management is similar. These patients are often intubated and propofol or midazolam is often titrated to the cessation of seizure activity; however, the treatment of these patients cannot be presented in an algorithm. There is still no data that aggressive treatment of non-convulsive seizures improves clinical outcomes; nevertheless, this is often done, unless the patient is only minimally symptomatic. When patients are minimally symptomatic, such as a patient in focal motor status (epilepsia partialis continua) the risk benefit ratio does not justify heavily sedating these patients to the point that they require mechanical ventilation.
In the general population, medication non-compliance is the most common cause of status epilepticus. While medication non-compliance is also a problem in the cancer population, there is a higher pretest probability that there is an underlying process causing the development of the refractory seizures, which needs to be identified and treated. For that reason, patients with cancer need an evaluation to look for a structural lesion, whether it is growth of a known brain tumor, a hemorrhage at the site of the tumor, worsening edema, or radiation necrosis. They also require an infectious work-up which may or may not include a lumbar puncture. In the case of seizures occurring in the setting of an enlarging tumor, worsening edema, or radiation necrosis, the administration of dexamethasone may be highly effective at improving seizure control.60
Stroke
Ischemic Stroke
Cancer causes hypercoagulability as it is an inflammatory state. Furthermore, certain tumors secrete procoagulant substances, and for these reasons, patients with cancer are at particularly high risk for ischemic stroke. A retrospective review at MSKCC found that adenocarcinomas, in particular, increase the risk, and that stroke was a risk factor for recurrent thromboembolic events (stroke, MI, and PE).61 Mechanisms by which many of these patients develop strokes based on autopsy studies include nonbacterial thrombotic endocarditis and disseminated intravascular coagulation.62 Patients with cancer are also at risk from stroke from the direct and indirect effects of chemotherapy and targeted treatments. Certain therapeutics are pro-thrombotic, such as bevacizumab, sunitinib, sorafenib, and cisplatin.52 Chemotherapy also promotes the development of stroke by compromising the immune system; thereby permitting the development of bacterial endocarditis, sepsis, and varicella zoster virus vasculopathy. Radiation to the head and neck, itself, increases the risk of TIA and ischemic stroke by at least two-fold with a median time to stroke of around 10 years. It does this by damaging the medium and large intra- and extra-cranial arteries and causing accelerated atherosclerosis.63 Other, rarer causes of stroke unique to the cancer population are tumor emboli from a cardiac metastasis, arterial occlusion by tumor, and PRES.
The management of stroke in the cancer population is similar to the management in the general population with certain nuances that will be discussed here. In the acute setting (within 4.5 hours of developing symptoms), patients with cancer should still be considered for intravenous tissue plasminogen activator (IV tPA). While a brain tumor was not an exclusion criterion for IV tPA in the original NINDS trial, the presence of an intracranial neoplasm has been added to the package insert as a contraindication.64 As there is a paucity of data, IV tPA may still be considered in patients with a non-vascular intracranial neoplasm, such as a meningioma or a vestibular schwannoma, after weighing the risk and benefits.65
Unfortunately, patients with cancer, even when they do not have brain tumors, often have other contraindications to IV TPA such as thrombocytopenia or recent major surgery. In 2014, the Multicenter Randomized Clinical Trial of Endovascular Treatment for Acute Ischemic Stroke in the Netherlands (the MR CLEAN trial) provided the first evidence that mechanical thrombectomy with the new stent retriever devices improves outcomes, when the intervention is performed within the first six hours.66 Since then, six additional trials have reported a similar benefit.67-70 Thrombectomy is not yet standard of care, but the data has matured and now there is strong evidence supporting its use. Therefore, patients with clinical evidence of a large acute stroke suggesting an accessible proximal clot should be evaluated for thrombectomy with a CT angiogram (CTA), MR angiogram (MRA), or catheter angiogram, as an adjunct to IV tPA and when IV tPA is contraindicated.
Even if patients do not have clinical evidence of a full MCA syndrome, a CTA or MRA may still be indicated because a proximal clot may be present and merely obscured by collateral circulation. Thrombectomy should be considered in patients who develop basilar artery occlusion even after 6 hours have elapsed from the last known normal, as the brainstem may be more resistant to ischemia, and strokes in this vascular distribution are particularly devastating.71
Intraparachymal Brain Hemorrhage
Intraparenchymal brain hemorrhage (IPH), like ischemic strokes, occur more frequently in the cancer population because chemotherapy and hematologic malignancies give rise to thrombocytopenia and coagulopathy, medications like bevacizumab predispose to hemorrhage, radiation vasculopathy can cause a moyamoya syndrome,72 and brain tumors spontaneously bleed. Among primary brain tumors, oligodendroglioma is the tumor type that is most prone to hemorrhage; among brain metastases, choriocarcinoma, melanoma, lung, papillary thyroid, and renal cell cancer are the primary tumor types that are most hemorrhagic.73
Acute promyelocytic leukemia (APL) is a hematologic malignancy that requires special mention as it accounts for a disproportionate number of IPH due to its ability to cause disseminated intravascular coagulation. APL is a highly curable disease with a cure rate that now exceeds 80%. Unfortunately, coagulopathy is present in 70-80% of these patients at the time of disease diagnosis. With improvement in supportive treatment, the incidence of IPH has dropped but it still occurs with relative regularity in cancer hospitals.74
The management of IPH in patients with cancer is similar to the management in the non-cancer population. It is important to correct coagulopathy and thrombocytopenia as soon as possible, and to lower blood pressure when the patient is hypertensive. A platelet goal of 100,000/microliter is typically chosen, but this may be very challenging to maintain because cancer patients frequently have a consumptive process and are frequently unable to produce platelets. Antiplatelet and anticoagulants should be held in the acute setting. While it is clear that coagulopathy should be corrected with fresh frozen plasma or prothrombin complex concentrate, it is unclear whether patients should be given platelet transfusions when they have received aspirin or medications like clopidogrel.75
The main indication for surgical intervention in hemorrhagic stroke is the development of hydrocephalus due to intraventricular extension of the IPH (necessitating an external ventricular device) and imminent herniation, which may be an indication for hemicraniectomy or suboccipital decompression.76 Clot evacuation was investigated in the Surgical Trials in Intracerebral Hemorrhage (STICH and STICH II) and was not shown to improve outcome; nevertheless, it is still considered in certain instances where the hematoma is superficial and the patient is deteriorating.77,78
In the general population, an investigation into the underlying cause frequently involves vascular imaging at the time of the hemorrhage with a CT angiogram or a MR angiogram. In the cancer population, in addition to the routine vascular imaging, it is often necessary to evaluate for a brain metastasis with a standard brain MRI in both the acute setting and a few months later after the hematoma has partially resorbed.
Venous thrombosis
In the International Study of Cerebral Venous and Dural Sinuses Thrombosis, 7.4% of cases of cerebral venous thrombosis were associated with cancer.79 Cancer patients are predisposed to thrombosis of their venous sinuses due to hypercoagulability but also because treatment of cancer can give rise to severe dehydration and certain medications can trigger thrombosis, such as the anti-estrogen tamoxifen, L-asparaginase, and heparin, specifically when it causes heparin induced thrombocytopenia (HIT).80,81 Venous sinus thrombosis can also occur due to venous compression by an extrinsic metastasis and rarely as a consequence of a bacterial infection, such as meningitis or an ear infection.
Venous sinus thrombosis, especially of the sagittal and lateral sinuses, results in headaches and the other symptoms of increased ICP. Individuals with venous sinus thrombosis also develop focal neurologic symptoms from ischemia and hemorrhagic strokes, as a consequence of venous congestion, and they may develop focal or generalized seizures.79 CT and MRI may suggest the presence of a venous sinus thrombosis by revealing evidence of a clot within the sinus or by identifying the sequela of venous congestion. However, those studies are frequently insufficient due to their lack of sensitivity and specificity. A study that permits direct visualization of the sinuses is the magnetic resonance venogram. CT venogram and conventional angiogram also image the venous system, but are infrequently used for this indication. Venous sinus thrombosis is a unique situation, where anticoagulation is the primary treatment even when there is evidence of IPH.79
CNS Infection
In patients with cancer, there should be a lower threshold for investigating meningitis with a lumbar puncture. In the general population, 44% of patients with bacterial meningitis had the classic triad of fever, neck stiffness, and altered mental status.82 Within the cancer population, only 5% have this same triad. In a retrospective study at MSKCC, the most common symptom was fever and was only present in 56% of the cohort.83 In neutropenic patients, a CSF pleocytosis is not always found; for this reason, these patients may require empiric CNS coverage until the CSF cultures have been negative for 48 hours. The causative organisms are quite different in the cancer population as the majority of infections are due to staphylococcus aureus and coagulase negative staphylococcus. This is a consequence of the high rates of infections associated with neurosurgical procedures. While vancomycin and ceftriaxone (with or without ampicillin—depending on the age and immunologic status of the patient) treats community acquired bacterial meningitis, in patients that have been in the hospital or undergone a neurosurgical procedure, coverage should include cefepime (vs ceftazidime or meropenem) rather than ceftriaxone.84
Viral encephalitides also occur much more frequently. Human herpesvirus 6 (HHV6) causes a limbic encephalitis in highly immunosuppressed individuals, most notably patients undergoing hematopoietic stem cell transplant. HHV6 encephalitis presents with headaches, seizures, amnesia, personality changes, and persistent cognitive deficits. The diagnosis is made by CSF PCR. On neuroimaging, the classic appearance of limbic encephalitis, T2 hyperintensity of the medial temporal lobes is typically seen 7 to 10 days after the development of symptoms. When the diagnosis is made, the antiviral medications used to treat the infection are foscarnet, ganciclovir or valganciclovir.85
The cancer population is also more susceptible to herpes simplex encephalitis, due to exposure to chemotherapy, corticosteroids and in patients with brain tumors, radiotherapy. Patients that are immunosuppressed, in addition to being more likely to have a bland CSF, are more likely to have a false negative on HSV PCR testing.85 For that reason, when the suspicion is high for HSV encephalitis—a patient with fever and a concerning MRI (T2/FLAIR hyperintensity, diffusion restriction, and enhancement of one or both temporal lobes)—it may be prudent to repeat the CSF HSV PCR testing prior to discontinuing acyclovir.86 Neuroinvasive West Nile Virus is another serious complication of chronic immunosuppression, and it has two presentations. One presentation is a poliomyelitis-like flaccid paralysis due to infection of the anterior horn cells, which can cause respiratory failure leading to intubation and mechanical ventilation. The other presentation is a meningitis or meningoencephalitis. A characteristic feature of West Nile Virus meningoencephalitis is a prominent movement disorder, which may include tremor, myoclonus, and Parkinsonism.87 The treatment is supportive.
Varicella re-activation should be considered in the immunocompromised patient with altered mental status and multifocal strokes that develop over time. Varicella can cause a small or large vessel vasculopathy, even in the absence of a Zoster outbreak or CSF pleocytosis. CSF VZV PCR is the most widely available test; however, CSF VZV IgG is more sensitive. The treatment is acyclovir and possibly prednisone to reduce the inflammatory component. VZV can also cause a meningoencephalitis or myelitis.88
Perhaps, the most devastating viral process that these patients develop is progressive multifocal leukoencephalopathy (PML)—a fatal demyelinating condition caused by the JC virus. Primary infection typically occurs during childhood, after which the JC virus becomes latent. In the setting of immunosuppression, the virus can become reactivated causing progressive neurologic deficits, such as hemiparesis, hemianopia, and aphasia. This disease achieved prominence in the 1980s, during the AIDS epidemic, but was first described in patients with cancer, specifically hematopoietic cancers, as a complication of CLL, Hodgkin Lymphoma, and Waldenstrom macroglobulinemia.89,90 PML can also occur in patients with hematologic malignancies on the order of months to over a year after stem cell transplant, and can be precipitated by immune modulating biologics such as the anti-CD20 antibody, rituximab, which results in B-cell depletion and prolonged dysfunction of the humoral immune response.91-93 On neuro-imaging PML causes focal or multifocal lesions that do not follow vascular territories as shown in figure 6. The lesions typically do not enhance and do not have surrounding edema, except when the individual is able to mount an inflammatory reaction, especially following immune reconstitution.94 There are no proven treatments other than reversing immune suppression.
Figure 6.
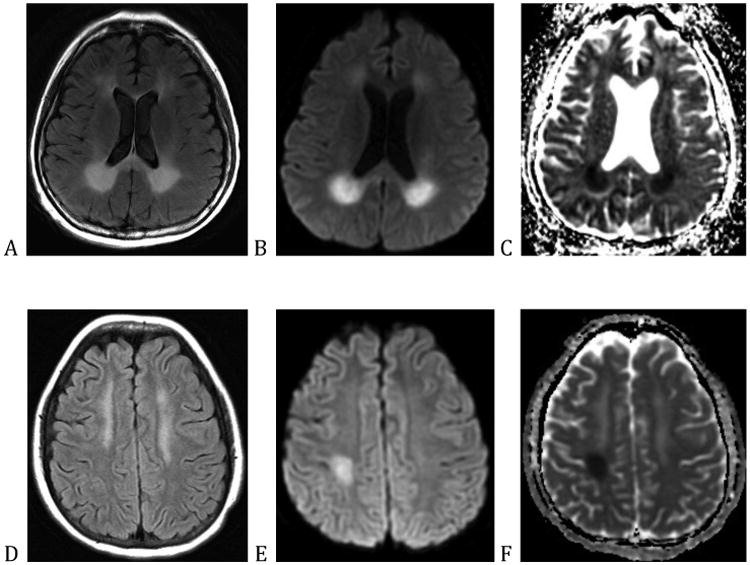
(A) through (C) are of a patient treated with fludarabine and (D) through (F) are of a patient treated with methotrexate. (A) and (D) are the fluid-attenuated inversion recovery (FLAIR) sequence, (B) and (E) are diffusion weighted imaging (DWI) sequences, and (C) and (F) are apparent diffusion coefficient (ADC) sequences.
Fungal meningitides such as cryptococcosis, blastomycosis and histoplasmosis, and parasitic infections like cerebral toxoplasmosis are the comparatively slow moving, deadly infections that are seen in these severely immunocompromised individuals.
Neurologic Complications of Cancer Treatment
Neurologic complications of cancer treatment is a broad topic, which cannot be discussed comprehensively in this review. Patients with cancer develop neurologic complication following radiotherapy, surgical procedures, and as a consequence of medications. This last category is very broad; one example, is PRES. As discussed, it can provoke seizures in patients with cancer, as well as cortical blindness, headache, and altered mental status. The diagnosis is made with imaging, typically MRI. While the parietal and occipital lobes are most commonly affected, the FLAIR/T2 hyperintensities are not necessarily posterior, the abnormality is not necessarily restricted to the white matter, and it is not always reversible (figure 7 A-C). It is not uncommon for there to be areas of diffusion restriction and even hemorrhage.52 The treatment is discontinuation of the offending drug and to make these patient normotensive as autoregulatory failure is central to its pathophysiology.
There are specific clinical syndromes caused by traditional cytotoxic chemotherapy. For example, ifosfamide causes a syndrome that includes hallucinations, personality changes, somnolence, agitation, confusion, seizures, cranial nerve palsies, and even coma, which occurs 12 hours to 6 days after treatment and is often self limited (but may be shortened by treated with methylene blue or thiamine).52,95,96 Cytarabine neurotoxicity may manifest as encephalopathy that evolves into a cerebellar syndrome that may not be reversible.96-99 Methotrexate neurotoxicity is another entity, which predominantly occurs in children, but can occur in adults following intravenous or intrathecal administration of the drug, and presents either acutely with confusion, headaches and seizure, or subacutely after several days with neurologic signs, such as dysarthria, hemiparesis, altered mental status, and seizures. On MRI, patients that develop subacute methotrexate neurotoxicity can show restricted diffusion, though the deficits can improve spontaneously over time (figure 6 D-F).1
Traditional cytotoxic chemotherapy is gradually being supplanted by biologics, small molecule inhibitors and immunotherapy, each with their own unique effects on the CNS and the peripheral nerves. Guillain-Barre Syndrome/chronic inflammatory demyelinating polyneuropathy (CIDP), myasthenia gravis, transverse myelitis and hypophysitis—resulting in cardiovascular collapse—have all been reported in patients receiving ipilimumab, a monoclonal antibody that increases the activity of the immune system by targeting CTLA-4, a receptor that downregulates the immune response.100 Genetically engineered T-cells with chimeric antigen receptors (CAR T-cells) is another form of immunotherapy that is being tested and has shown significant promise. CAR T-cells are designed to engage tumor cells in leukemia, lymphoma, and certain solid tumors, and has an unusual complication known as cytokine release syndrome, which manifests as fevers, hypotension, and hypoxia. It causes severe neurologic deficits ranging from encephalopathy to obtundation, in the presence or absence of seizure activity.101
Conclusion
Neurologic complications of cancer are diverse and a common cause of morbidity and mortality in the cancer hospital. They are frequently emergencies, which start insidiously, making the diagnosis challenging. A high index of suspicion is often needed to make diagnoses such as increased ICP, hydrocephalus, subclinical seizures, meningitis, venous sinus thrombosis, and cord compression. Failure to recognize a developing neurologic complication can be catastrophic since immediate treatment is often required to ensure the best possible neurologic outcome.
Oncology is an exciting field as new treatments are constantly being developed that offer new hope to patients with cancer. As the treatment of cancer evolves, so does the spectrum of neuro-oncologic emergencies.
Acknowledgments
We thank Wichit Sae-Ow, MD for sharing the image of the histologic appearance of radiation necrosis and Judith Lampron for her editorial assistance.
Grants: None.
References
- 1.DeAngelis LM, Posner JB, Posner JB. Neurologic complications of cancer. 2nd. Oxford; New York: Oxford University Press; 2009. [Google Scholar]
- 2.Gilbert MR, Grossman SA. Incidence and nature of neurologic problems in patients with solid tumors. Am J Med. 1986;81:951–954. doi: 10.1016/0002-9343(86)90385-2. [DOI] [PubMed] [Google Scholar]
- 3.Smith ER, Madsen JR. Cerebral pathophysiology and critical care neurology: basic hemodynamic principles, cerebral perfusion, and intracranial pressure. Semin Pediatr Neurol. 2004;11:89–104. doi: 10.1016/j.spen.2004.04.001. [DOI] [PubMed] [Google Scholar]
- 4.Mokri B. The Monro-Kellie hypothesis: applications in CSF volume depletion. Neurology. 2001;56:1746–1748. doi: 10.1212/wnl.56.12.1746. [DOI] [PubMed] [Google Scholar]
- 5.Stocchetti N, Maas AI, Chieregato A, van der Plas AA. Hyperventilation in head injury: a review. Chest. 2005;127:1812–1827. doi: 10.1378/chest.127.5.1812. [DOI] [PubMed] [Google Scholar]
- 6.Gower DJ, Baker AL, Bell WO, Ball MR. Contraindications to lumbar puncture as defined by computed cranial tomography. J Neurol Neurosurg Psychiatry. 1987;50:1071–1074. doi: 10.1136/jnnp.50.8.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brandes AA, Tosoni A, Spagnolli F, et al. Disease progression or pseudoprogression after concomitant radiochemotherapy treatment: pitfalls in neurooncology. Neuro Oncol. 2008;10:361–367. doi: 10.1215/15228517-2008-008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Perry A, Schmidt RE. Cancer therapy-associated CNS neuropathology: an update and review of the literature. Acta Neuropathol. 2006;111:197–212. doi: 10.1007/s00401-005-0023-y. [DOI] [PubMed] [Google Scholar]
- 9.Hygino da Cruz LC, Jr, Rodriguez I, Domingues RC, Gasparetto EL, Sorensen AG. Pseudoprogression and pseudoresponse: imaging challenges in the assessment of posttreatment glioma. AJNR Am J Neuroradiol. 2011;32:1978–1985. doi: 10.3174/ajnr.A2397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Forsyth PA, Posner JB. Headaches in patients with brain tumors: a study of 111 patients. Neurology. 1993;43:1678–1683. doi: 10.1212/wnl.43.9.1678. [DOI] [PubMed] [Google Scholar]
- 11.Hayashi M, Kobayashi H, Handa Y, Kawano H, Kabuto M. Brain blood volume and blood flow in patients with plateau waves. J Neurosurg. 1985;63:556–561. doi: 10.3171/jns.1985.63.4.0556. [DOI] [PubMed] [Google Scholar]
- 12.Magnaes B. Body position and cerebrospinal fluid pressure. Part 1: clinical studies on the effect of rapid postural changes. J Neurosurg. 1976;44:687–697. doi: 10.3171/jns.1976.44.6.0687. [DOI] [PubMed] [Google Scholar]
- 13.Azarmina M, Azarmina H. The six syndromes of the sixth cranial nerve. J Ophthalmic Vis Res. 2013;8:160–171. [PMC free article] [PubMed] [Google Scholar]
- 14.Kalmar AF, Van Aken J, Caemaert J, Mortier EP, Struys MM. Value of Cushing reflex as warning sign for brain ischaemia during neuroendoscopy. Br J Anaesth. 2005;94:791–799. doi: 10.1093/bja/aei121. [DOI] [PubMed] [Google Scholar]
- 15.Agrawal A, Timothy J, Cincu R, Agarwal T, Waghmare LB. Bradycardia in neurosurgery. Clin Neurol Neurosurg. 2008;110:321–327. doi: 10.1016/j.clineuro.2008.01.013. [DOI] [PubMed] [Google Scholar]
- 16.Koenig MA, Bryan M, Lewin JL, 3rd, Mirski MA, Geocadin RG, Stevens RD. Reversal of transtentorial herniation with hypertonic saline. Neurology. 2008;70:1023–1029. doi: 10.1212/01.wnl.0000304042.05557.60. [DOI] [PubMed] [Google Scholar]
- 17.Marino R, Gasparotti R, Pinelli L, et al. Posttraumatic cerebral infarction in patients with moderate or severe head trauma. Neurology. 2006;67:1165–1171. doi: 10.1212/01.wnl.0000238081.35281.b5. [DOI] [PubMed] [Google Scholar]
- 18.Safavi-Abbasi S, Maurer AJ, Archer JB, et al. From the notch to a glioma grading system: the neurological contributions of James Watson Kernohan. Neurosurg Focus. 2014;36:E4. doi: 10.3171/2014.1.FOCUS13575. [DOI] [PubMed] [Google Scholar]
- 19.Parizel PM, Makkat S, Jorens PG, et al. Brainstem hemorrhage in descending transtentorial herniation (Duret hemorrhage) Intensive Care Med. 2002;28:85–88. doi: 10.1007/s00134-001-1160-y. [DOI] [PubMed] [Google Scholar]
- 20.Peterson K, Forsyth PA, Posner JB. Paraneoplastic sensorimotor neuropathy associated with breast cancer. J Neurooncol. 1994;21:159–170. doi: 10.1007/BF01052900. [DOI] [PubMed] [Google Scholar]
- 21.Giacino JT, Smart CM. Recent advances in behavioral assessment of individuals with disorders of consciousness. Curr Opin Neurol. 2007;20:614–619. doi: 10.1097/WCO.0b013e3282f189ef. [DOI] [PubMed] [Google Scholar]
- 22.Woessner H, Vibhute P, Barrett K. Acute loss of bladder control in a stroke of the frontal cortex. Neurohospitalist. 2012;2:129–131. doi: 10.1177/1941874412450715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cuneo RA, Caronna JJ, Pitts L, Townsend J, Winestock DP. Upward transtentorial herniation: seven cases and a literature review. Arch Neurol. 1979;36:618–623. doi: 10.1001/archneur.1979.00500460052006. [DOI] [PubMed] [Google Scholar]
- 24.Ryken TC, McDermott M, Robinson PD, et al. The role of steroids in the management of brain metastases: a systematic review and evidence-based clinical practice guideline. J Neurooncol. 2010;96:103–114. doi: 10.1007/s11060-009-0057-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kolmodin L, Sekhon MS, Henderson WR, Turgeon AF, Griesdale DE. Hypernatremia in patients with severe traumatic brain injury: a systematic review. Ann Intensive Care. 2013;3:35. doi: 10.1186/2110-5820-3-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Muizelaar JP, Marmarou A, Ward JD, et al. Adverse effects of prolonged hyperventilation in patients with severe head injury: a randomized clinical trial. J Neurosurg. 1991;75:731–739. doi: 10.3171/jns.1991.75.5.0731. [DOI] [PubMed] [Google Scholar]
- 27.Boothe D, Young R, Yamada Y, Prager A, Chan T, Beal K. Bevacizumab as a treatment for radiation necrosis of brain metastases post stereotactic radiosurgery. Neuro Oncol. 2013;15:1257–1263. doi: 10.1093/neuonc/not085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Slone HW, Blake JJ, Shah R, Guttikonda S, Bourekas EC. CT and MRI findings of intracranial lymphoma. AJR Am J Roentgenol. 2005;184:1679–1685. doi: 10.2214/ajr.184.5.01841679. [DOI] [PubMed] [Google Scholar]
- 29.Pope WB, Lai A, Nghiemphu P, Mischel P, Cloughesy TF. MRI in patients with high-grade gliomas treated with bevacizumab and chemotherapy. Neurology. 2006;66:1258–1260. doi: 10.1212/01.wnl.0000208958.29600.87. [DOI] [PubMed] [Google Scholar]
- 30.Dietrich J, Rao K, Pastorino S, Kesari S. Corticosteroids in brain cancer patients: benefits and pitfalls. Expert Rev Clin Pharmacol. 2011;4:233–242. doi: 10.1586/ecp.11.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vredenburgh JJ, Desjardins A, Herndon JE, 2nd, et al. Phase II trial of bevacizumab and irinotecan in recurrent malignant glioma. Clin Cancer Res. 2007;13:1253–1259. doi: 10.1158/1078-0432.CCR-06-2309. [DOI] [PubMed] [Google Scholar]
- 32.Levin VA, Bidaut L, Hou P, et al. Randomized double-blind placebo-controlled trial of bevacizumab therapy for radiation necrosis of the central nervous system. Int J Radiat Oncol Biol Phys. 2011;79:1487–1495. doi: 10.1016/j.ijrobp.2009.12.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ananthnarayan S, Bahng J, Roring J, et al. Time course of imaging changes of GBM during extended bevacizumab treatment. J Neurooncol. 2008;88:339–347. doi: 10.1007/s11060-008-9573-x. [DOI] [PubMed] [Google Scholar]
- 34.de la Fuente MI, DeAngelis LM. The role of ventriculoperitoneal shunting in patients with supratentorial glioma. Ann Clin Transl Neurol. 2014;1:45–48. doi: 10.1002/acn3.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Clarke JL. Leptomeningeal metastasis from systemic cancer. Continuum (Minneap Minn) 2012;18:328–342. doi: 10.1212/01.CON.0000413661.58045.e7. [DOI] [PubMed] [Google Scholar]
- 36.Omuro AM, Lallana EC, Bilsky MH, DeAngelis LM. Ventriculoperitoneal shunt in patients with leptomeningeal metastasis. Neurology. 2005;64:1625–1627. doi: 10.1212/01.WNL.0000160396.69050.DC. [DOI] [PubMed] [Google Scholar]
- 37.Rubinstein LJ, Herman MM, Long TF, Wilbur JR. Disseminated necrotizing leukoencephalopathy: a complication of treated central nervous system leukemia and lymphoma. Cancer. 1975;35:291–305. doi: 10.1002/1097-0142(197502)35:2<291::aid-cncr2820350202>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 38.Pigott KH, Baddeley H, Maher EJ. Pattern of disease in spinal cord compression on MRI scan and implications for treatment. Clin Oncol (R Coll Radiol) 1994;6:7–10. doi: 10.1016/s0936-6555(05)80361-9. [DOI] [PubMed] [Google Scholar]
- 39.Hammack JE. Spinal cord disease in patients with cancer. Continuum (Minneap Minn) 2012;18:312–327. doi: 10.1212/01.CON.0000413660.58045.ae. [DOI] [PubMed] [Google Scholar]
- 40.Levack P, Graham J, Collie D, et al. Don't wait for a sensory level--listen to the symptoms: a prospective audit of the delays in diagnosis of malignant cord compression. Clin Oncol (R Coll Radiol) 2002;14:472–480. doi: 10.1053/clon.2002.0098. [DOI] [PubMed] [Google Scholar]
- 41.Perry JR, Deodhare SS, Bilbao JM, Murray D, Muller P. The significance of spinal cord compression as the initial manifestation of lymphoma. Neurosurgery. 1993;32:157–162. doi: 10.1227/00006123-199302000-00001. [DOI] [PubMed] [Google Scholar]
- 42.Gemici C. Lhermitte's sign: Review with special emphasis in oncology practice. Crit Rev Oncol Hematol. 2010;74:79–86. doi: 10.1016/j.critrevonc.2009.04.009. [DOI] [PubMed] [Google Scholar]
- 43.Laird AS, Finch AM, Waite PM, Carrive P. Peripheral changes above and below injury level lead to prolonged vascular responses following high spinal cord injury. Am J Physiol Heart Circ Physiol. 2008;294:H785–792. doi: 10.1152/ajpheart.01002.2007. [DOI] [PubMed] [Google Scholar]
- 44.Vecht CJ, Haaxma-Reiche H, van Putten WL, de Visser M, Vries EP, Twijnstra A. Initial bolus of conventional versus high-dose dexamethasone in metastatic spinal cord compression. Neurology. 1989;39:1255–1257. doi: 10.1212/wnl.39.9.1255. [DOI] [PubMed] [Google Scholar]
- 45.Patchell RA, Tibbs PA, Regine WF, et al. Direct decompressive surgical resection in the treatment of spinal cord compression caused by metastatic cancer: a randomised trial. Lancet. 2005;366:643–648. doi: 10.1016/S0140-6736(05)66954-1. [DOI] [PubMed] [Google Scholar]
- 46.Loblaw DA, Perry J, Chambers A, Laperriere NJ. Systematic review of the diagnosis and management of malignant extradural spinal cord compression: the Cancer Care Ontario Practice Guidelines Initiative's Neuro-Oncology Disease Site Group. J Clin Oncol. 2005;23:2028–2037. doi: 10.1200/JCO.2005.00.067. [DOI] [PubMed] [Google Scholar]
- 47.Glantz MJ, Cole BF, Forsyth PA, et al. Practice parameter: anticonvulsant prophylaxis in patients with newly diagnosed brain tumors. Report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2000;54:1886–1893. doi: 10.1212/wnl.54.10.1886. [DOI] [PubMed] [Google Scholar]
- 48.Kerkhof M, Vecht CJ. Seizure characteristics and prognostic factors of gliomas. Epilepsia. 2013;54(9):12–17. doi: 10.1111/epi.12437. [DOI] [PubMed] [Google Scholar]
- 49.Van Breemen MS, Wilms EB, Vecht CJ. Seizure control in brain tumors. Handb Clin Neurol. 2012;104:381–389. doi: 10.1016/B978-0-444-52138-5.00026-8. [DOI] [PubMed] [Google Scholar]
- 50.Lowenstein DH, Alldredge BK. Status epilepticus. N Engl J Med. 1998;338:970–976. doi: 10.1056/NEJM199804023381407. [DOI] [PubMed] [Google Scholar]
- 51.Bartynski WS. Posterior reversible encephalopathy syndrome, part 1: fundamental imaging and clinical features. AJNR Am J Neuroradiol. 2008;29:1036–1042. doi: 10.3174/ajnr.A0928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lee EQ, Arrillaga-Romany IC, Wen PY. Neurologic complications of cancer drug therapies. Continuum (Minneap Minn) 2012;18:355–365. doi: 10.1212/01.CON.0000413663.42798.64. [DOI] [PubMed] [Google Scholar]
- 53.Castilla-Guerra L, del Carmen Fernandez-Moreno M, Lopez-Chozas JM, Fernandez-Bolanos R. Electrolytes disturbances and seizures. Epilepsia. 2006;47:1990–1998. doi: 10.1111/j.1528-1167.2006.00861.x. [DOI] [PubMed] [Google Scholar]
- 54.Drislane FW. Nonconvulsive status epilepticus in patients with cancer. Clin Neurol Neurosurg. 1994;96:314–318. doi: 10.1016/0303-8467(94)90121-x. [DOI] [PubMed] [Google Scholar]
- 55.Treiman DM, Meyers PD, Walton NY, et al. A comparison of four treatments for generalized convulsive status epilepticus. Veterans Affairs Status Epilepticus Cooperative Study Group. N Engl J Med. 1998;339:792–798. doi: 10.1056/NEJM199809173391202. [DOI] [PubMed] [Google Scholar]
- 56.Silbergleit R, Durkalski V, Lowenstein D, et al. Intramuscular versus intravenous therapy for prehospital status epilepticus. N Engl J Med. 2012;366:591–600. doi: 10.1056/NEJMoa1107494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Prasad M, Krishnan PR, Sequeira R, Al-Roomi K. Anticonvulsant therapy for status epilepticus. Cochrane Database Syst Rev. 2014;9:CD003723. doi: 10.1002/14651858.CD003723.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Browne TR, Kugler AR, Eldon MA. Pharmacology and pharmacokinetics of fosphenytoin. Neurology. 1996;46:S3–7. doi: 10.1212/wnl.46.6_suppl_1.3s. [DOI] [PubMed] [Google Scholar]
- 59.Brophy GM, Bell R, Claassen J, et al. Guidelines for the evaluation and management of status epilepticus. Neurocrit Care. 2012;17:3–23. doi: 10.1007/s12028-012-9695-z. [DOI] [PubMed] [Google Scholar]
- 60.Araki T, Otsubo H, Makino Y, et al. Efficacy of dexamathasone on cerebral swelling and seizures during subdural grid EEG recording in children. Epilepsia. 2006;47:176–180. doi: 10.1111/j.1528-1167.2006.00384.x. [DOI] [PubMed] [Google Scholar]
- 61.Navi BB, Singer S, Merkler AE, et al. Recurrent thromboembolic events after ischemic stroke in patients with cancer. Neurology. 2014;83:26–33. doi: 10.1212/WNL.0000000000000539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Graus F, Rogers LR, Posner JB. Cerebrovascular complications in patients with cancer. Medicine (Baltimore) 1985;64:16–35. doi: 10.1097/00005792-198501000-00002. [DOI] [PubMed] [Google Scholar]
- 63.Plummer C, Henderson RD, O'Sullivan JD, Read SJ. Ischemic stroke and transient ischemic attack after head and neck radiotherapy: a review. Stroke. 2011;42:2410–2418. doi: 10.1161/STROKEAHA.111.615203. [DOI] [PubMed] [Google Scholar]
- 64.Tissue plasminogen activator for acute ischemic stroke. The National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group. N Engl J Med. 1995;333:1581–1587. doi: 10.1056/NEJM199512143332401. [DOI] [PubMed] [Google Scholar]
- 65.Neil W, Ovbiagele B. Intravenous thrombolysis in ischemic stroke patients with intracranial neoplasms: two cases and a literature review. Case Rep Med. 2011;2011:503758. doi: 10.1155/2011/503758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Fransen PS, Beumer D, Berkhemer OA, et al. MR CLEAN, a multicenter randomized clinical trial of endovascular treatment for acute ischemic stroke in the Netherlands: study protocol for a randomized controlled trial. Trials. 2014;15:343. doi: 10.1186/1745-6215-15-343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Campbell BC, Mitchell PJ, Kleinig TJ, et al. Endovascular Therapy for Ischemic Stroke with Perfusion-Imaging Selection. N Engl J Med. 2015 doi: 10.1056/NEJMoa1414792. [DOI] [PubMed] [Google Scholar]
- 68.Goyal M, Demchuk AM, Menon BK, et al. Randomized Assessment of Rapid Endovascular Treatment of Ischemic Stroke. N Engl J Med. 2015 doi: 10.1056/NEJMoa1414905. [DOI] [PubMed] [Google Scholar]
- 69.Jovin TG, Chamorro A, Cobo E, et al. Thrombectomy within 8 hours after symptom onset in ischemic stroke. N Engl J Med. 2015;372:2296–2306. doi: 10.1056/NEJMoa1503780. [DOI] [PubMed] [Google Scholar]
- 70.Saver JL, Goyal M, Bonafe A, et al. Stent-retriever thrombectomy after intravenous t-PA vs. t-PA alone in stroke. N Engl J Med. 2015;372:2285–2295. doi: 10.1056/NEJMoa1415061. [DOI] [PubMed] [Google Scholar]
- 71.Pfefferkorn T, Mayer TE, Opherk C, et al. Staged escalation therapy in acute basilar artery occlusion: intravenous thrombolysis and on-demand consecutive endovascular mechanical thrombectomy: preliminary experience in 16 patients. Stroke. 2008;39:1496–1500. doi: 10.1161/STROKEAHA.107.505123. [DOI] [PubMed] [Google Scholar]
- 72.Ullrich NJ, Robertson R, Kinnamon DD, et al. Moyamoya following cranial irradiation for primary brain tumors in children. Neurology. 2007;68:932–938. doi: 10.1212/01.wnl.0000257095.33125.48. [DOI] [PubMed] [Google Scholar]
- 73.Bernstein M, Berger MS. Neuro-oncology : the essentials. Third [Google Scholar]
- 74.Chen CY, Tai CH, Tsay W, Chen PY, Tien HF. Prediction of fatal intracranial hemorrhage in patients with acute myeloid leukemia. Ann Oncol. 2009;20:1100–1104. doi: 10.1093/annonc/mdn755. [DOI] [PubMed] [Google Scholar]
- 75.Martin M, Conlon LW. Does platelet transfusion improve outcomes in patients with spontaneous or traumatic intracerebral hemorrhage? Ann Emerg Med. 2013;61:58–61. doi: 10.1016/j.annemergmed.2012.03.025. [DOI] [PubMed] [Google Scholar]
- 76.Heuts SG, Bruce SS, Zacharia BE, et al. Decompressive hemicraniectomy without clot evacuation in dominant-sided intracerebral hemorrhage with ICP crisis. Neurosurg Focus. 2013;34:E4. doi: 10.3171/2013.2.FOCUS1326. [DOI] [PubMed] [Google Scholar]
- 77.Mendelow AD, Gregson BA, Rowan EN, et al. Early surgery versus initial conservative treatment in patients with spontaneous supratentorial lobar intracerebral haematomas (STICH II): a randomised trial. Lancet. 2013;382:397–408. doi: 10.1016/S0140-6736(13)60986-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Mendelow AD, Gregson BA, Fernandes HM, et al. Early surgery versus initial conservative treatment in patients with spontaneous supratentorial intracerebral haematomas in the International Surgical Trial in Intracerebral Haemorrhage (STICH): a randomised trial. Lancet. 2005;365:387–397. doi: 10.1016/S0140-6736(05)17826-X. [DOI] [PubMed] [Google Scholar]
- 79.Saposnik G, Barinagarrementeria F, Brown RD, Jr, et al. Diagnosis and management of cerebral venous thrombosis: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2011;42:1158–1192. doi: 10.1161/STR.0b013e31820a8364. [DOI] [PubMed] [Google Scholar]
- 80.Finelli PF, Schauer PK. Cerebral sinus thrombosis with tamoxifen. Neurology. 2001;56:1113–1114. doi: 10.1212/wnl.56.8.1113-a. [DOI] [PubMed] [Google Scholar]
- 81.Ranta S, Tuckuviene R, Makipernaa A, et al. Cerebral sinus venous thromboses in children with acute lymphoblastic leukaemia - a multicentre study from the Nordic Society of Paediatric Haematology and Oncology. Br J Haematol. 2014 doi: 10.1111/bjh.13162. [DOI] [PubMed] [Google Scholar]
- 82.van de Beek D, de Gans J, Spanjaard L, Weisfelt M, Reitsma JB, Vermeulen M. Clinical features and prognostic factors in adults with bacterial meningitis. N Engl J Med. 2004;351:1849–1859. doi: 10.1056/NEJMoa040845. [DOI] [PubMed] [Google Scholar]
- 83.Safdieh JE, Mead PA, Sepkowitz KA, Kiehn TE, Abrey LE. Bacterial and fungal meningitis in patients with cancer. Neurology. 2008;70:943–947. doi: 10.1212/01.wnl.0000305960.85546.5f. [DOI] [PubMed] [Google Scholar]
- 84.Tunkel AR, Hartman BJ, Kaplan SL, et al. Practice guidelines for the management of bacterial meningitis. Clin Infect Dis. 2004;39:1267–1284. doi: 10.1086/425368. [DOI] [PubMed] [Google Scholar]
- 85.Bhanushali MJ, Kranick SM, Freeman AF, et al. Human herpes 6 virus encephalitis complicating allogeneic hematopoietic stem cell transplantation. Neurology. 2013;80:1494–1500. doi: 10.1212/WNL.0b013e31828cf8a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sili U, Kaya A, Mert A Group HSVES. Herpes simplex virus encephalitis: clinical manifestations, diagnosis and outcome in 106 adult patients. J Clin Virol. 2014;60:112–118. doi: 10.1016/j.jcv.2014.03.010. [DOI] [PubMed] [Google Scholar]
- 87.Davis LE, DeBiasi R, Goade DE, et al. West Nile virus neuroinvasive disease. Ann Neurol. 2006;60:286–300. doi: 10.1002/ana.20959. [DOI] [PubMed] [Google Scholar]
- 88.Nagel MA, Gilden D. The challenging patient with varicella-zoster virus disease. Neurol Clin Pract. 2013;3:109–117. doi: 10.1212/CPJ.0b013e31828d9f92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Carson KR, Evens AM, Richey EA, et al. Progressive multifocal leukoencephalopathy after rituximab therapy in HIV-negative patients: a report of 57 cases from the Research on Adverse Drug Events and Reports project. Blood. 2009;113:4834–4840. doi: 10.1182/blood-2008-10-186999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ng C, Slavin MA, Seymour JF. Progressive multifocal leukoencephalopathy complicating Waldenstrom's macroglobulinaemia. Leuk Lymphoma. 2003;44:1819–1821. doi: 10.1080/1042819031000111071. [DOI] [PubMed] [Google Scholar]
- 91.Kaufman GP, Aksamit AJ, Klein CJ, Yi ES, Delone DR, Litzow MR. Progressive multifocal leukoencephalopathy: a rare infectious complication following allogeneic hematopoietic cell transplantation (HCT) Eur J Haematol. 2014;92:83–87. doi: 10.1111/ejh.12208. [DOI] [PubMed] [Google Scholar]
- 92.D'Souza A, Wilson J, Mukherjee S, Jaiyesimi I. Progressive multifocal leukoencephalopathy in chronic lymphocytic leukemia: a report of three cases and review of the literature. Clin Lymphoma Myeloma Leuk. 2010;10:E1–9. doi: 10.3816/CLML.2010.n.009. [DOI] [PubMed] [Google Scholar]
- 93.Clifford DB, Ances B, Costello C, et al. Rituximab-associated progressive multifocal leukoencephalopathy in rheumatoid arthritis. Arch Neurol. 2011;68:1156–1164. doi: 10.1001/archneurol.2011.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Sahraian MA, Radue EW, Eshaghi A, Besliu S, Minagar A. Progressive multifocal leukoencephalopathy: a review of the neuroimaging features and differential diagnosis. Eur J Neurol. 2012;19:1060–1069. doi: 10.1111/j.1468-1331.2011.03597.x. [DOI] [PubMed] [Google Scholar]
- 95.Buesa JM, Garcia-Teijido P, Losa R, Fra J. Treatment of ifosfamide encephalopathy with intravenous thiamin. Clin Cancer Res. 2003;9:4636–4637. [PubMed] [Google Scholar]
- 96.Pelgrims J, De Vos F, Van den Brande J, Schrijvers D, Prove A, Vermorken JB. Methylene blue in the treatment and prevention of ifosfamide-induced encephalopathy: report of 12 cases and a review of the literature. Br J Cancer. 2000;82:291–294. doi: 10.1054/bjoc.1999.0917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Damon I, Murphy PM, Moss B. Broad spectrum chemokine antagonistic activity of a human poxvirus chemokine homolog. Proc Natl Acad Sci U S A. 1998;95:6403–6407. doi: 10.1073/pnas.95.11.6403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Szawarski P, Chapman CS. A woman who couldn't speak: report of methotrexate neurotoxicity. Postgrad Med J. 2005;81:194–195. doi: 10.1136/pgmj.2003.018267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hayashi M, Kobayashi H, Handa Y, Kawano H, Hirose S, Ishii H. Plateau-wave phenomenon (II). Occurrence of brain herniation in patients with and without plateau waves. Brain. 1991;114(Pt 6):2693–2699. doi: 10.1093/brain/114.6.2693. [DOI] [PubMed] [Google Scholar]
- 100.Liao B, Shroff S, Kamiya-Matsuoka C, Tummala S. Atypical neurological complications of ipilimumab therapy in patients with metastatic melanoma. Neuro Oncol. 2014;16:589–593. doi: 10.1093/neuonc/nou001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Davila ML, Riviere I, Wang X, et al. Efficacy and toxicity management of 19-28z CAR T cell therapy in B cell acute lymphoblastic leukemia. Sci Transl Med. 2014;6:224ra225. doi: 10.1126/scitranslmed.3008226. [DOI] [PMC free article] [PubMed] [Google Scholar]