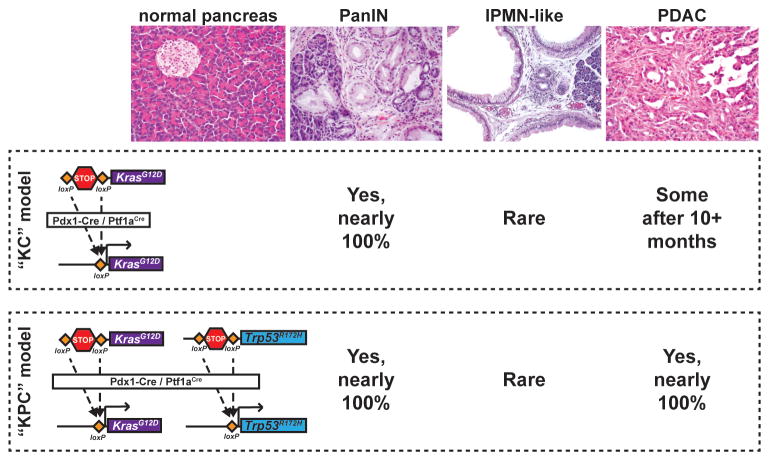
Figure 2.
Mouse Models of Pancreatic Ductal Adenocarcinoma (PDAC) Replicate Human Pathology. To activate cancer-causing alleles specifically within the mouse pancreatic epithelium, Cre recombinase is expressed under the control of the Pdx1 or Ptf1a/p48 promoter, both of which are active in embryonic pancreatic progenitor cells. In the ‘KC’ (Kras/Cre) model, one copy of the endogenous Kras allele is mutated to change amino acid 12 from glycine to aspartic acid, locking the Kras protein in its GTP-bound (active) form. A loxP–STOP–loxP cassette (LSL) is placed upstream of this mutation and, before recombination, inhibits transcription of the mutant allele. When Cre is expressed it excises the STOP cassette, activating KrasG12D in all epithelial cells of the early pancreas. This produces fully-penetrant pancreatic intraepithelial neoplasia (PanIN) formation, rare intraductal papillary mucinous neoplasia (IPMN) formation, and occasional PDAC (in ~50% of aged animals). In the ‘KPC’ (Kras/p53/Cre) model of invasive PDAC, the same oncogenic Kras allele is activated along with a Trp53 ‘gain-of-function’ (neomorphic) allele. The R172H point mutation in this Trp53 allele models a mutation frequently found in human PDAC. Expression of both KrasG12D and p53R172H using Pdx1–Cre results in fully-penetrant PanIN and PDAC development in young animals.