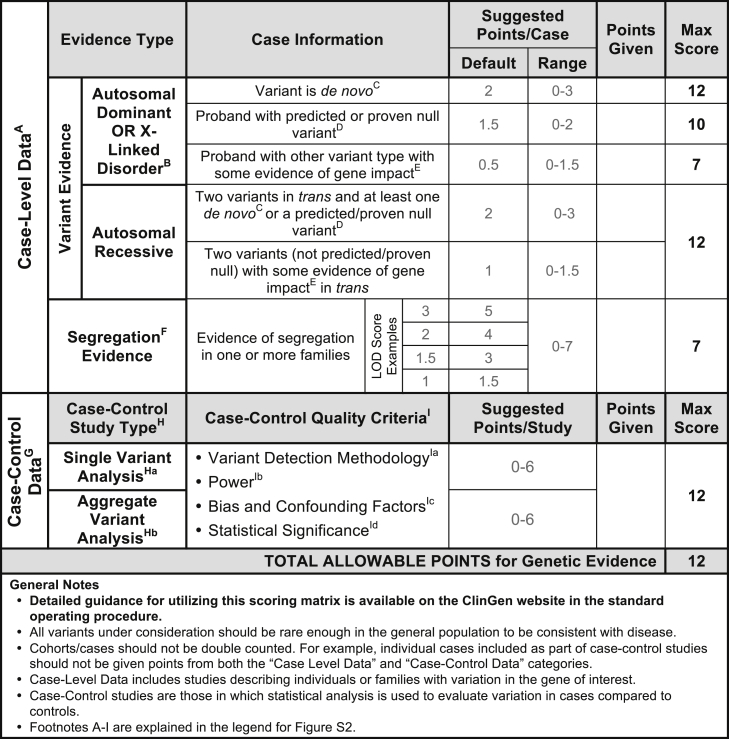
Figure 2.
Classes of Genetic Evidence and Their Relative Weights Used in the ClinGen Clinical Validity Framework
For additional points to consider when scoring genetic evidence, please see the standard operating procedure document available on our website. Genetic evidence is separated into two main categories: case-level data and case-control data. While a single publication may include both case-level and case-control data, individual cases should NOT be included in both categories. Each category is assigned a range of points with a maximum score that can be achieved. Case-level data are derived from studies describing individuals and/or families with qualifying variants in the gene of interest. Points should be assigned to each case based on the variant’s inheritance pattern, molecular consequence, and evidence of pathogenicity in disease. In addition to variant evidence points, a gene-disease pair may also receive points for compelling segregation analysis (see Figure S1). Case-Control Data: Studies utilizing statistical analysis to evaluate variants in case subjects compared to control subjects. Case-control studies can be classified as either single-variant analysis or aggregate variant analysis, but the number of points allowable for either category is the same. Points should be assigned according to the overall quality of each study based on these criteria: variant detection methodology, power, bias and confounding factors, and statistical power. Note that the maximum total scores allowed for different types of case-level data are not intended to add up to the total points allowed for genetic evidence as a whole. This permits different combinations of evidence types to achieve the maximum total score.