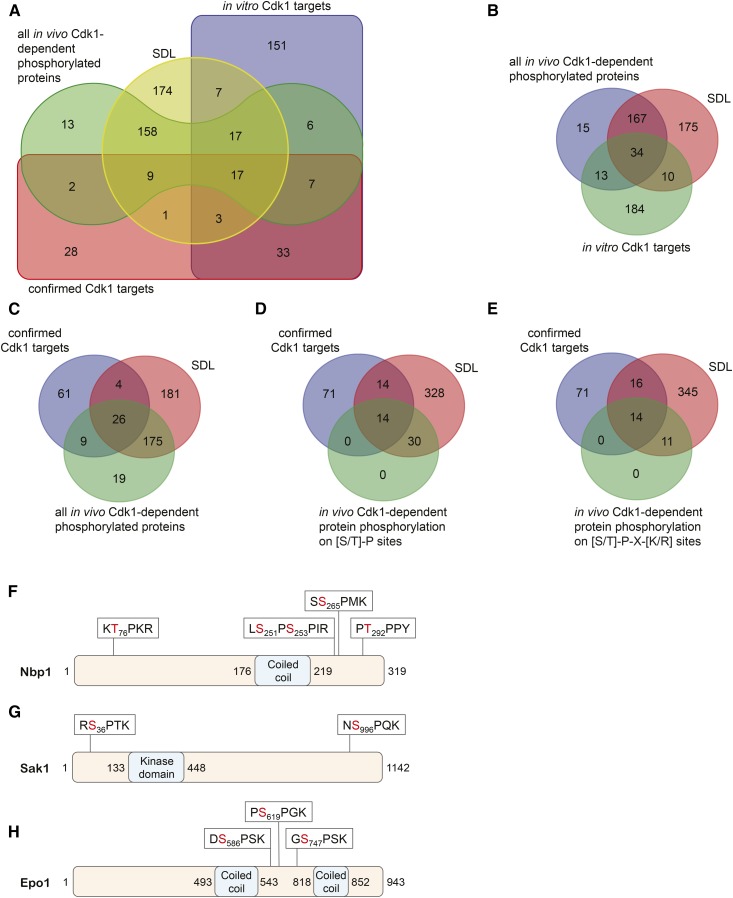
Figure 7.
The SDL dataset is enriched for proteins that are phosphorylated in a Cdc28-dependent manner. (A) Venn diagram depicting overlap between the SDL dataset and proteins that are phosphorylated in a Cdc28-dependent manner in vivo and in vitro, and which have previously been confirmed to be Cdc28 substrates. (B) Most proteins that have been found to be phosphorylated in a Cdc28-dependent manner in vivo cause dosage lethality when overexpressed in a cdc28-as1 mutant. The Venn diagram shows overlap between the SDL dataset and proteins that are phosphorylated in a Cdc28-dependent manner in vitro and in vivo. (C) The SDL dataset is enriched for known Cdc28 targets. The Venn diagram shows overlap between the SDL screen, proteins that have been found to be phosphorylated in a Cdc28-dependent manner in vivo, and previously confirmed Cdc28 substrates. (D and E) Overexpression of proteins phosphorylated on potential Cdc28 sites cause an SDL phenotype. The Venn diagrams show overlap between the SDL dataset, confirmed Cdc28 targets, and proteins that are known to be phosphorylated in vivo on either minimal Cdc28 sites (D) or on full Cdc28 consensus sites (E) in a Cdc28-dependent manner. (F–H) Domain structures of Nbp1 (F), Sak1 (G), and Epo1 (H), and location of sites known to be phosphorylated in vivo in a Cdc28-dependent manner. CDK, cyclin-dependent kinase; SDL, synthetic dosage lethality.