Figure 1.
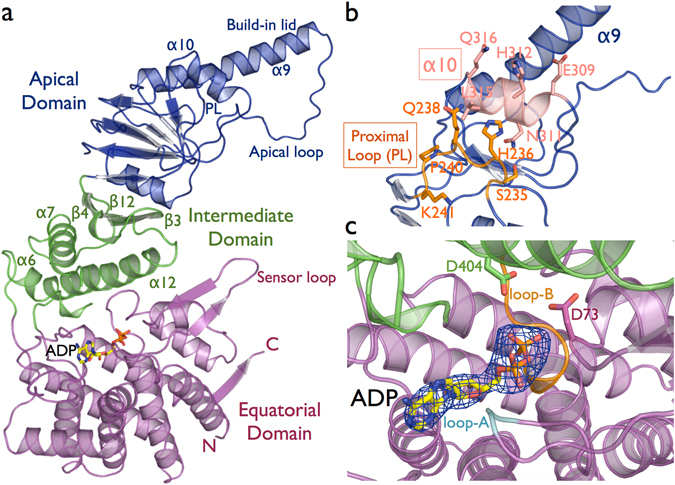
(a) Cartoon representation of human CCT5 subunit architecture in complex with ADP nucleotide. The N-terminal and C-terminal regions of the subunit form the equatorial domain, which includes the nucleotide-binding site and sensor loop. The intermediate domain that connects the equatorial and apical domains via the lower hinge is composed of α6, α7 and α12, and the upper hinge represented by the β-sheet β3/β4/β12. The apical domain is associated with the substrate binding recognition and with the substrate-folding process though the apical loop region. In addition, the extended α9 serves as a lid to close the TRiC ring chamber. (b) The substrate-binding interface has been identified between the α10 helix in and the proximal loop (PL) region28. In CCT5, the α10 is formed by the residues 309EANHLLLQ316 and the proximal loop by the residues 234FSHPQMPK241, with surface-exposed residues represented in sticks. (c) A mFo-DFc electron density map omitting the ADP nucleotide molecule contoured at 3.0 σ is shown in blue. The nucleotide-binding site shows the loop-A and the loop-B surrounding the phosphate groups of ADP. The ATP hydrolysis occurs by a water nucleophilic attack, and the water molecule is held in place by the represented residues D73 and D404.
