Figure 1.
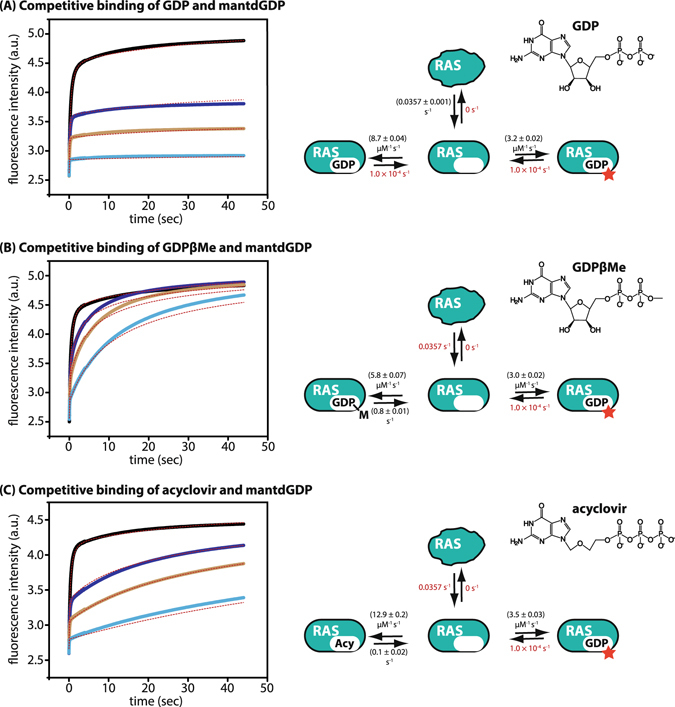
Interaction of KRas with different nucleotides. In order to quantify the interaction of nucleotide-free KRas, competitive binding experiments between mantdGDP and GDP (A), GDPβMe (B) and acyclovir triphosphate (C) were performed. In a stopped-flow apparatus, 1 µM KRas was shot against 1 µM mantdGDP in the absence (black curve) or in the presence of 0.5 µM (blue), 1 µM (orange) or 3 µM (turquoise) competing nucleotide and the binding curves were globally fit to the model on the right of the diagram to obtain the corresponding binding constants (indicated in black, previously known kinetic constants that were fixed during fitting are indicated in red). In all experiments, a second slow phase was observed that could only be explained by a model in which nucleotide-free KRas is in an equilibrium between a competent (70–80%) and a non-competent (20–30%) nucleotide-binding state, which might arise from partly unfolded protein due to the inherent instability of KRas in the absence of nucleotides.
