Figure 2.
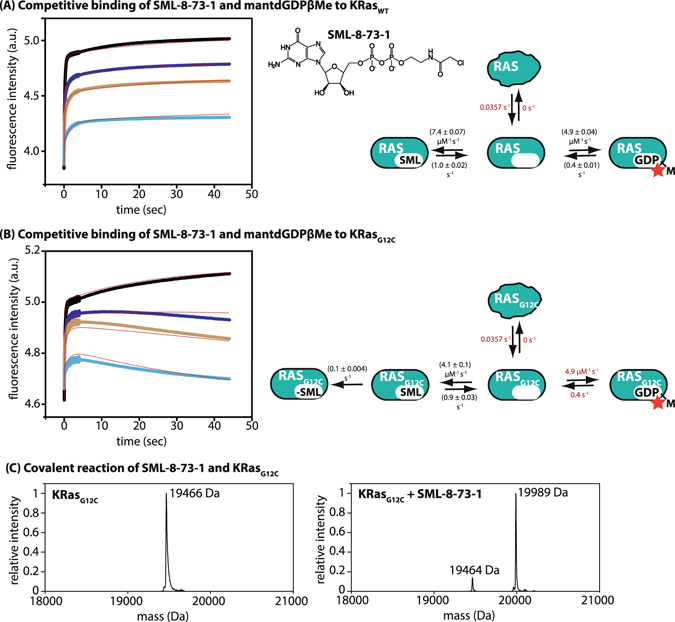
Reversible and irreversible interaction of the inhibitor SML-8-73-1 with KRas. (A) In order to quantify the reversible binding of SML-8-73-1, competition experiments of KRasWT binding to mantdGDPβMe in the absence (black curve) or in the presence of 0.5 µM (blue), 1 µM (orange) or 3 µM (turquoise) SML-8-73-1 were performed and kinetic constants were obtained by global fitting of the data to the model on the right. (B) Irreversible binding was subsequently quantified by repeating the experiments from 2 A, but using KRasG12C instead of the wild type protein. The additional phase observed in these experiments in the presence of SML-8-73-1 corresponding to displacement of initially bound mantdGDPβMe because of covalent reaction of the inhibitor allowed determination of the rate constant of covalent reaction of SML-8-73-1 with KRasG12C. (C) Covalent reaction was confirmed by mass spectrometry. The mass spectra of nucleotide-free KRasG12C before (left) and after (right) addition of SML-8-73-1 and quenching with 5 mM DTE after 20 s are shown.
