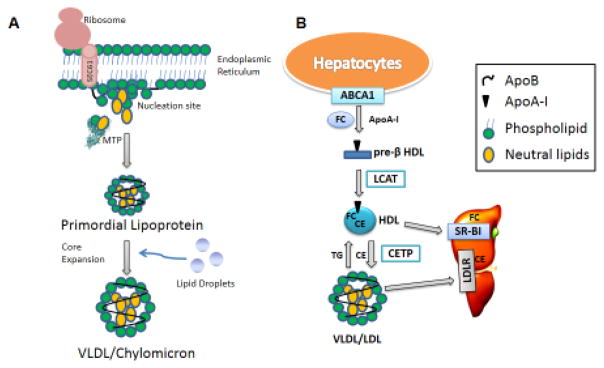
Figure 1. Lipoprotein Assembly.
(A) VLDL and chylomicron follow similar biosynthesis pathways in the liver and intestine, respectively. While apoB is being translated, MTP physically binds apoB to possibly help it translocate into the endoplasmic reticulum lumen and to provide structural stability for further lipidation. As hydrophobic sequences are translated, apoB associates with the lumenal side of the ER membrane to form nucleation sites. MTP may transfer cholesteryl esters, triglycerides, free cholesterol, ceramide, and sphingomyelin to these nucleation sites and the translating apoB to form primordial lipoproteins. In the second step of lipoprotein assembly, the primordial lipoproteins fuse with lumenal lipid droplets to form VLDL or chylomicron that are secreted into the blood and lymph, respectively. (B) HDL Assembly. Apolipoprotein A-I (apoA-I) interacts with plasma membrane-embedded ABCA1 and accepts free cholesterol (FC) to form pre-β HDL extracellularly. Lecithin – cholesterol acyltransferase (LCAT) converts FC in pre-β HDL to cholesteryl esters (CE), which move to the core of HDL. HDL’s CE may be selectively delivered to the liver or steroidogenic tissues by scavenger receptor BI (SR-BI). Cholesteryl ester transfer protein (CETP) exchanges CE for triglycerides from HDL to apoB-containing lipoproteins. CE transferred to apoB-containing lipoproteins are delivered to the liver via receptor mediated endocytosis involving receptors such as LDL receptors.