Abstract
Background & Aims
Proton pump inhibitors (PPIs) have been associated with increased risk of infection, likely due to changes in intestinal epithelial permeability and the gastrointestinal microbiome. PPIs are frequently given to patients in the intensive care unit (ICU) to prevent stress ulcers. These patients are at risk for bloodstream infections (BSIs), so we investigated the relationship between PPI use and BSIs among patients in the ICU.
Methods
We performed a retrospective cohort study of adults (≥18 years) admitted to 1 of 14 ICUs within a hospital network of 3 large hospitals from 2008 through 2014. The primary exposure was PPI use for stress ulcer prophylaxis in the ICU. The primary outcome was BSI, confirmed by culture analysis, arising 48 hrs or more after admission to the ICU. Subjects were followed for 30 days after ICU admission or until death, discharge, or BSI. Multivariable Cox proportional-hazards modeling was used to test the association between PPIs and BSI, after controlling for patient comorbidities and other clinical factors.
Results
We analyzed data from 24,774 patients in the ICU, including 756 patients (3.1%) who developed BSIs while in the ICU. The cumulative incidence of BSI was 3.7% in patients with PPI exposure compared to 2.2% in patients without PPI exposure (log-rank test P<.01). After adjusting for potential confounders, PPI exposure was not associated with increased risk of BSI while in the ICU (adjusted hazard ratio, 1.08; 95% CI, 0.91–1.29). Comorbidities, antibiotic use, and mechanical ventilation were all independently associated with increased risk for BSIs.
Conclusion
In a retrospective study of patients in the ICU, administration of PPIs to prevent bleeding was not associated with increased risk of BSI. These findings indicate that concern for BSI should not affect decisions regarding use of PPIs in the ICU.
Keywords: Acid suppression, intestinal permeability, critical care, bacteremia
INTRODUCTION
There are 600,000 bacterial bloodstream infections annually in the United States, over 75,000 of which result in death.1 Bloodstream infections are particularly lethal when acquired in the intensive care unit (ICU) with a case mortality rate over 40%, a 15–20% absolute increase above baseline ICU mortality.2 Established risk factors for bloodstream infection (BSI) in the ICU include increased age, multiple comorbidities, and immunosuppression.3 Potentially modifiable risk factors include indwelling venous or urinary catheters,4 hygiene and other local environmental factors,5, 6 and receipt of antibiotics and other medications.7 Comprehensive strategies to decrease the incidence of ICU-acquired BSI have been effective8 although incidence and mortality from BSIs in the ICU remain high.9
Proton pump inhibitors (PPIs) are frequently used for prophylaxis against upper gastrointestinal (GI) bleeding in the ICU and are highly effective for this purpose.10 Current guidelines recommend use of PPIs for stress ulcer prophylaxis in ICU patients with characteristics that place them at high risk for bleeding such as sepsis, extended mechanical ventilation, or coagulopathy.11 Because most ICU patients have these high-risk characteristics, use of PPIs in the critical care setting is very common. As long as PPIs cause few adverse effects, widespread use of PPIs in the ICU is likely to be of net benefit.12
There is reason for concern that PPIs may be associated with increased risk for infection and that this association may be particularly strong in critically ill patients. Most notably, PPIs have been associated with increased risk for BSIs and for other infections in cirrhotics.13, 14 Like cirrhotics, critically ill patients have crucial risk factors for BSI that may be affected by PPIs including increased intestinal permeability15 and loss of normal diversity in the gastrointestinal microbiome.16, 17 Data investigating the relationship between PPIs and risk for BSI is limited. To evaluate this relationship further, we performed a retrospective cohort study among patients hospitalized in the ICU.
METHODS
The institutional review board of Columbia University approved this study.
Data sources
Data was extracted from the hospital electronic medical record (EMR) using algorithms validated for the assessment of healthcare-associated infections and that have previously been described.18 In brief, diverse sources of electronic data were combined into a single, curated repository that included patient demographics, comorbidities (computed as the Charlson Comorbidity Index (CCI)),19 provider order entry data, microbiology results, and insurance claims data. This repository was then queried for relevant outcome and exposure-related data.
Study population
Adult patients (≥18 years) were eligible for the study if they were admitted to any one of 14 distinct ICUs within a hospital network comprised of three large hospitals between 2008 and 2014. Patients were excluded if they had an ICU length of stay less than two days, if they were diagnosed with BSI prior to day three of their ICU stay (in order to distinguish prevalent from incident BSI), or if they had GI bleeding during the index hospitalization (identified by appropriate ICD-9 codes). GI bleeding is the main indication for use of PPIs other than stress ulcer prophylaxis and we wished to focus the study on use of PPIs for bleeding prophylaxis rather than for management of bleeding. For those with multiple ICU admissions during the study period, only data from the first admission was analyzed.
Bloodstream infections
Bloodstream infection was classified as present if a blood culture drawn either peripherally or centrally showed bacterial growth on or after the third ICU day. Otherwise, BSI was classified as absent. This criterion aligns with guidelines from the Centers for Disease Control and Prevention, which define healthcare-associated infections as those arising a minimum of 48 hours after hospitalization.20 BSI onset was considered to be the time that the positive blood culture was collected. To focus on the possible mechanisms linking PPIs and BSI, we sub-classified BSIs as derived from predominantly enteric bacteria (Bacteroides, Enterococcus, Fusobacterium and the Enterobacteriaceae family of gram negatives including common pathogens Citrobacter, Enterobacter, Escherichia coli, Klebsiella, Proteus, Salmonella and Serratia) or derived from predominantly non-enteric bacteria (e.g. Staphylococcus aureus).21
Primary exposure
The primary exposure was receipt of PPIs at any dose or duration, either oral or intravenous, at any time during the follow-up period in the ICU, and was classified as present or absent. Exposure to PPIs was extracted through the EMR from nursing flowsheets (i.e. based on when PPIs were actually administered) and was classified as present only if PPIs were received at least one day prior to the last day of follow-up.
Covariates
The following variables were extracted: age, sex, race, residence in a long-term care facility prior to hospital admission, history of solid organ transplant, and baseline comorbidities (CCI).19 Additionally, data was extracted related to ICU exposures: use of hemodialysis, use of gastrostomy tube, mechanical ventilation, major surgery, presence of a central venous catheter or urinary catheter, receipt of histamine-2 receptor antagonists (H2RAs), and receipt of antibiotics in the ICU. Receipt of H2RAs and antibiotics was classified as present if these drugs were given at any dose or duration a minimum of one day prior to the last day of follow-up. Antibiotics were further sub-classified as narrow-spectrum or broad-spectrum based on their anticipated impact on the gastrointestinal microbiome.22 In situations where the spectrum of the antibiotic class was controversial,23 it was categorized as narrow-spectrum if that class lacked significant activity against anaerobes (e.g., monobactam antibiotics). CCI was dichotomized as a score of 2 or less, considered mild, versus 3 or greater, considered moderate to severe.24
Statistical analysis
Descriptive statistics were visualized to determine appropriate cut-offs and chi-squared tests were used to compare categorical variables. The final multivariable analysis was performed using a Cox proportional hazards model with patients followed from the time of ICU admission until death, ICU discharge, or for 28 total days (i.e., from day 2 until day 30 after ICU admission). The proportionality assumption was verified based on visual inspection and by testing for a non-zero slope in the Schoenfield residuals. We decided a priori that the following variables represented important potential confounders for the PPI-BSI relationship and would be forced into the final model: age, presence of a central venous catheter in the ICU, exposure to antibiotics, and baseline comorbidities. Additional variables were tested stepwise in the model and included if they were independently associated with BSI or if they changed the β-coefficient representing PPIs by ≥10%. Statistical analyses were performed using STATA version 14.1 and statistical significance was defined as p-value of <0.05.
Sensitivity analyses
To assess the possibility of death as a competing risk for BSI, we performed a stratified analysis based on death. We assessed for a dose-response relationship between PPIs and BSIs and also assessed PPIs as a time-varying exposure (i.e., patients were coded as unexposed until the day that they received a PPI and as exposed thereafter). To evaluate whether the PPI-BSI relationship depended on the presence or absence of antibiotics, we tested for interactions between PPIs and narrow- and broad-spectrum antibiotics. Last, because the hypothesized mechanism linking PPIs and BSI involves the translocation of gut bacteria across the intestinal wall, we tested whether exposure to PPIs was a risk factor for infections derived from predominantly enteric bacteria.
Because concerns have been raised regarding PPIs and risk for ventilator-associated pneumonia (VAP), we also extracted data related to the presence or absence of VAPs within this cohort. VAP was classified as present in mechanically ventilated patients who had moderate to heavy bacterial growth from sputum cultures or fluid taken during bronchoalveolar lavage. Because VAP is frequently culture-negative, ventilated patients were also classified as having VAPs if they were coded with ICD-9 or ICD-10 codes for pneumonia. When ventilated patients had neither positive cultures nor appropriate ICD codes, VAP was classified as absent. Because ICD codes did not identify the date on which VAP occurred, logistic regression modeling rather than a Cox model was used to test for an association between PPIs and VAP after adjusting for potential confounders.
RESULTS
Study population
From 60,764 patients initially evaluated for the study, 24,774 patients met eligibility criteria and were included in the analysis. A total of 756 patients (3.1%) developed blood stream infection between day 3 and 30 of ICU stay. The mortality rate during the index ICU admission was 19% among patients who developed BSIs compared to 7.7% among patients who did not develop BSIs (p<0.01). The incidence rate of BSIs was similar throughout the seven years of the study (p for trend=0.26).
Characteristics at baseline and during treatment in the ICU
Patients who received prophylaxis with PPIs were older, more likely to be male, more likely to have received a solid organ transplant, and had increased baseline comorbidities compared to patients who did not receive PPIs (Table 1). During treatment in the ICU, patients who received PPIs were more likely to receive antibiotics and other interventions compared to patients who did not receive PPIs (Table 2).
Table 1.
Baseline demographics and characteristics at the time of ICU admission, stratified by exposure to PPIs.
| Characteristics | All (n=24,774) | No PPIs (n=10,134) | PPIs (n=14,640) | P-value |
|---|---|---|---|---|
| Sex | ||||
| Male | 13,423 (46%) | 5,412 (47%) | 8,011 (55%) | .04 |
| Female | 11,351 (54%) | 4,722 (53%) | 6,629 (45%) | |
| Age | ||||
| Under 45 | 4,559 (18%) | 2,285 (23%) | 2,274 (16%) | <.01 |
| 45–65 | 8,583 (35%) | 3,423 (34%) | 5,160 (35%) | |
| 65+ | 11,632 (47%) | 4,426 (44%) | 7,206 (49%) | |
| Race | ||||
| White | 7,602 (31%) | 3,051 (30%) | 4,551 (31%) | .25 |
| Black | 1,809 (7.3%) | 751 (7.4%) | 1,058 (7.2%) | |
| Hispanic/Unspecified | 15,363 (62%) | 6,332 (62%) | 9,031 (62%) | |
| Longterm care* | ||||
| No | 23,921 (97%) | 9,801 (97%) | 14,120 (96%) | .26 |
| Yes | 853 (3.4%) | 333 (3.3%) | 520 (3.6%) | |
| Hosp floor admit | ||||
| No | 16,343 (66%) | 6,716 (66%) | 9,627 (66%) | .40 |
| Yes | 8,431 (34%) | 3,418 (34%) | 5,013 (34%) | |
| Organ transplant | ||||
| No | 24,064 (97%) | 10,018 (99%) | 14,046 (96%) | .26 |
| Yes | 710 (2.9%) | 116 (1.1%) | 594 (4.1%) | |
| CCI | ||||
| 0–2 points | 14,609 (59%) | 6,485 (64%) | 8,124 (55%) | <.01 |
| ≥3 points | 10,165 (41%) | 3,649 (36%) | 6,516 (45%) | |
| Comorbidities | ||||
| Pulm disorders | 9,189 (37%) | 3,296 (33%) | 5,893 (40%) | <.01 |
| Diabetes Mellitus | 6,156 (25%) | 2,373 (23%) | 3,783 (26%) | <.01 |
| Renal failure | 9,475 (38%) | 3,265 (32%) | 6,210 (42%) | <.01 |
| Malignancy | 3,996 (16%) | 1,515 (15%) | 2,481 (17%) | <.01 |
Residence prior to hospitalization in a longterm care facility; ICU, intensive care unit; PPIs, proton pump inhibitors; Pulm Disorders, Chronic Pulmonary Disorders.
Table 2.
Characteristics during treatment in the ICU, stratified by exposure to PPIs.
| Characteristics | All (n=24,774) | No PPIs (n=10,134) | PPIs (n=14,640) | P-value |
|---|---|---|---|---|
| Antibiotics | ||||
| None | 13,368 (54%) | 6,356 (63%) | 7,012 (48%) | <.01 |
| Narrow | 5,673 (23%) | 1,835 (18%) | 3,838 (26%) | |
| Broad | 5,733 (23%) | 1,943 (19%) | 3,790 (26%) | |
| Mechanical ventilation | ||||
| No | 17,944 (72%) | 7,910 (78%) | 10,034 (69%) | <.01 |
| Yes | 6,830 (28%) | 2,224 (22%) | 4,606 (31%) | |
| Hemodialysis | ||||
| No | 23,266 (94%) | 9,698 (96%) | 13,568 (93%) | <.01 |
| Yes | 1,508 (6.1%) | 436 (4.3%) | 1,072 (7.3%) | |
| Central venous catheter | ||||
| No | 10,969 (44%) | 5,757 (57%) | 5,212 (36%) | <.01 |
| Yes | 13,805 (56%) | 4,377 (43%) | 9,428 (64%) | |
| Urinary catheter | ||||
| No | 6,295 (25%) | 3,478 (34%) | 2,817 (19%) | <.01 |
| Yes | 18,479 (75%) | 6,656 (66%) | 11,823 (81%) | |
| PEG | ||||
| No | 23,314 (94%) | 9,695 (96%) | 13,619 (93%) | <.01 |
| Yes | 1,460 (5.9%) | 439 (4.3%) | 1,021 (7.0%) | |
| Major surgery | ||||
| No | 15,511 (63%) | 7,259 (72%) | 8,252 (56%) | <.01 |
| Yes | 9,263 (37%) | 2,875 (28%) | 6,388 (44%) | |
PPIs, proton pump inhibitors; PEG, Percutaneous Endoscopic Gastrostomy Tube; GI bleeding, gastrointestinal bleeding; ICU, intensive care unit.
Multivariable model
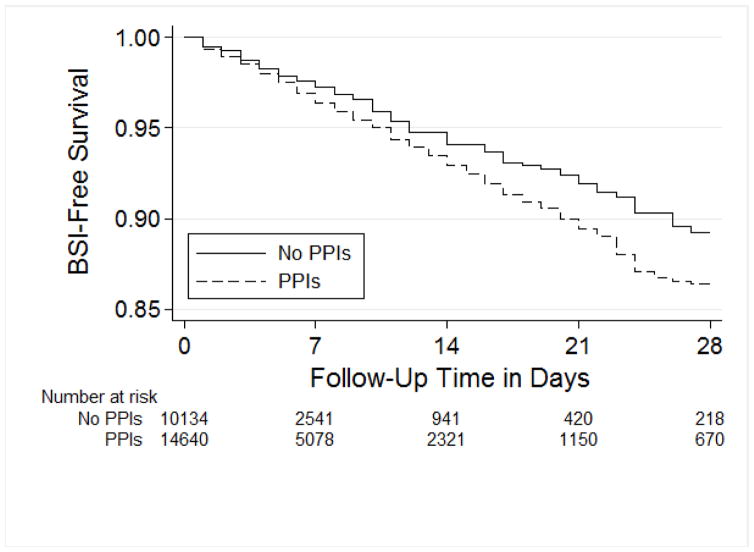
The cumulative proportion of BSIs was 3.7% in patients exposed to PPIs versus 2.2% in patients who were not exposed to PPIs (log-rank test P<0.01, Figure 1). However, after adjusting for potential confounders, there was no association between PPIs and bloodstream infections (aHR 1.08, 95% CI 0.91–1.29 Table 3). When tested in the final model, exposure to H2RAs in the ICU was also not significantly associated with BSI (aHR 1.01, 95% CI 0.74–1.38). Increased comorbidities, use of mechanical ventilation, and receipt of narrow- and broad-spectrum antibiotics were independently associated with BSI in the ICU. Within antibiotics, a stronger association with BSI was observed with broad-spectrum antibiotics (aHR 2.44, 95% CI 2.00–2.96) compared to narrow-spectrum antibiotics (aHR 1.70, 95% CI 1.36–2.13).
Figure 1.
Table 3.
Final Cox proportional hazards model of risk factors for ICU-onset blood stream infections.
| Risk Factors | BSI/Total Exposed (%) | Hazard Ratio (95% CI) |
|---|---|---|
| PPIs | ||
| No | 222 / 10,134 (2.2%) | Ref |
| Yes | 534 / 14,640 (3.7%) | 1.08 (0.91–1.29) |
| H2RAs only | ||
| No | 704 / 23,343 (3.0%) | Ref |
| Yes | 52 / 1,431 (3.6%) | 1.01 (0.74–1.38) |
| Age category | ||
| Under 45 | 115 / 4,559 (2.5%) | Ref |
| 45–65 | 288 / 8,583 (3.4%) | 1.21 (0.97–1.50) |
| 65+ | 353 / 11,632 (3.0%) | 1.23 (0.99–1.52) |
| CCI | ||
| 0–2 | 379 / 14,609 (2.6%) | Ref |
| 3+ | 377 / 10,165 (3.7%) | 1.17 (1.01–1.35) |
| Antibiotics | ||
| None | 169 / 13,368 (1.3%) | Ref |
| Narrow spectrum | 156 / 5,673 (2.8%) | 1.70 (1.36–2.13) |
| Broad spectrum | 431 / 5,733 (7.5%) | 2.44 (2.00–2.96) |
| Central venous catheter | ||
| No | 180 / 10,969 (1.6%) | Ref |
| Yes | 576 / 13,805 (4.2%) | 1.08 (0.89–1.31) |
| Mechanical ventilation | ||
| No | 336 / 17,944 (1.9%) | Ref |
| Yes | 420 / 6,830 (6.2%) | 1.42 (1.21–1.66) |
ICU, intensive care unit; BSI, bloodstream infections; CI, confidence interval; PPIs, proton pump inhibitors; H2RAs, histamine-2 receptor antagonists; HR, Hazard Ratio; CCI, Charlson Comorbidity Index.
Sensitivity analyses
The relationship between PPIs and BSI remained unchanged when we performed a restriction analysis of 22,776 patients who survived until ICU discharge or the development of BSI (aHR 1.04, 95% CI 0.86–1.27). Results of a Fine-Gray analysis were similar (aHR 1.11, 95% CI 0.93–1.34). There was also no difference when we focused on the sickest patients by excluding 9,738 patients with fewer than two serious medical comorbidities at the time of ICU admission (aHR 0.96, 95% CI 0.79–1.18). Among patients who received at least a single dose of a PPI, 88% received PPIs for more than 50% of ICU days. When patients who received PPIs for <50% of ICU days were excluded, there was no association between PPIs and BSI (aHR 1.13, 95% CI 0.94–1.35). When PPIs were examined as a time-varying exposure, there was no association with BSI (aHR 1.16, 95% CI 0.97–1.39). There was no interaction between PPIs and either broad-spectrum antibiotics (p=0.92) or narrow-spectrum antibiotics (p=0.39). There was also no significant association between PPIs and BSI when we analyzed only 203 BSIs that were derived from predominantly enteric bacteria (aHR 1.35, 95% CI 0.94–1.95). Finally, there was no association between PPIs and VAP (aOR 1.16, 95% CI 0.97–1.39, Supplemental Table 1) or PPIs and all-cause mortality (aHR 1.01, 95% CI 0.92–1.10).
DISCUSSION
Use of PPIs for stress ulcer prophylaxis in the ICU was not associated with increased risk for BSI, and this null finding was robust through multiple sensitivity analyses. If the relationship between PPIs and BSI was mediated by PPI-induced changes in intestinal permeability, one might expect that PPIs would increase risk for BSI from enteric but not from non-enteric bacteria. However, there was also no association between PPIs and risk for BSI with predominantly enteric organisms. Comorbidities, antibiotics, and mechanical ventilation were the chief factors influencing risk for ICU-onset BSI. In addition, PPIs were not associated with VAP nor with overall ICU mortality.
This is the first study to examine the relationship between PPIs and risk for bloodstream infections. Prior studies related to PPIs and risk for systemic infections have focused on cirrhotics and risk for spontaneous bacterial peritonitis (SBP). In a meta-analysis by Xu et al., PPI use was associated with 2-fold increased risk for both SBP and overall bacterial infections.14 In hospitalized cirrhotics, long-term PPI use was an independent predictor of subsequent SBP and all-cause infections.13 Alterations in the gastrointestinal mucosal barrier and in the gastrointestinal microbiome in cirrhotic patients are thought to underlie this increased risk.25 Critically ill patients have similar underlying risk factors for infections. Animal and human studies have shown higher intestinal permeability with slower rates of mucosal healing in the critically ill leading to increased risk for bacterial translocation and infection.15, 26 Because most of the data connecting PPIs and extra-intestinal infections is derived from cirrhotics, the differences between this study and prior studies may indicate that ICU patients have relatively preserved intestinal permeability compared to cirrhotics and that BSI in ICU patients usually arises through alternate pathways. There were too few cirrhotics in this study to test whether cirrhosis modified the PPI-BSI relationship.
Direct evidence that PPIs alter intestinal barrier function is contradictory. In animal models and in humans, PPIs appear to exacerbate small bowel injury due to NSAIDS.27, 28 On the other hand, Jones et al. performed gastroduodenoscopy with biopsy on healthy dogs with or without PPIs, and found that bacteremia after endoscopy was rare, and did not depend on PPI exposure.29 Lumenal micro-organisms contribute towards mucosal integrity through diverse mechanisms.30 Because PPIs appear to alter the human gastrointestinal microbiome,31–33 it has been hypothesized that they may have a detrimental effect on mucosal barrier function that is mediated by the gut microbiota. PPIs have also been found to lead to increases in pathogenic taxa as well as genes involved in bacterial invasion.31, 34 This study indirectly addressed the question of PPIs and gut barrier function in the ICU. Along with previous studies, these data suggest that PPIs do not meaningfully alter clinical outcomes with respect to some of the most important ICU infections: bloodstream infections, VAP, or Clostridium difficile infection.35 In the ICU setting, any effects of PPIs may simply be too subtle or too short-lived to exert a downstream effect on risk for BSI or other infections.
Receipt of both narrow- and broad-spectrum antibiotics in the ICU was associated with increased risk for BSI, with a stronger association observed for broad- than narrow-spectrum antibiotics. Previous studies have found that prior antibiotic exposure (within the past 90 days) leads to increased in-hospital mortality from Gram-negative bacterial infection.7 Prior use of broad-spectrum antibiotics has been associated with increased risk for ICU-acquired infection.36 Our findings are consistent with these previous studies and emphasize the potential for antibiotics to impact long-term risk for infections by altering the gastrointestinal microbiome, immune function, bacterial antibiotic resistance, or other mechanisms.37
There are limitations to the current study. This was a large study, but we cannot completely exclude the possibility that use of PPIs for stress ulcer prophylaxis is associated with a modest increase in risk for BSI. With regard to the study outcome, only culture-proven BSIs were included in our analysis. Assessment of infection without culture results in the ICU can be subjective and the requirement for positive culture results may thus minimize the possibility of bias. We were unable to retrospectively assess the indication for PPIs. However, the primary indications for PPI use in the ICU are for stress ulcer prophylaxis and for GI bleeding and patients with bleeding were excluded from the study. We did not have data on immunosuppressant use, which may impact individual patients’ susceptibility to infection. Our study did take into account organ transplant recipients, the majority of whom are on immunosuppressive agents, and this did not impact the relationship between PPIs and ICU-onset BSI. Finally, in order to focus on incident rather than prevalent BSI, we excluded patients who died within 48 hours of ICU admission. As a result, our cohort may have been healthier than other ICU-based cohorts.38
In sum, use of PPIs for stress ulcer prophylaxis was not a significant predictor of BSI or VAP in this large, ICU-based cohort. There was no association between PPIs and BSI due to predominantly enteric bacteria, and also no PPI-BSI relationship in subjects who received PPIs for a majority of their ICU days. Exposure to both narrow- and broad-spectrum antibiotics was associated with increased risk of ICU-onset BSI with a stronger association observed for broad-spectrum antibiotics; the potential for antibiotics to increase risk for BSI and other infections merits further study. Our null findings regarding PPIs provide important reassurance that concern for BSIs should not drive the decision regarding whether or not to use PPIs for stress ulcer prophylaxis in the ICU. More generally, our findings do not support the hypothesis that PPIs significantly alter intestinal permeability to predispose to BSIs or other infections.
Supplementary Material
Acknowledgments
Grant support: Dr. Freedberg was supported by a Research Scholar Award from the American Gastroenterological Association (AGA) and by the National Center for Advancing Translational Sciences, National Institutes of Health (NIH), (KL2 TR000081, formerly KL2 RR024157). The views expressed in this article represent those of the authors and do not necessarily represent the views of the AGA or the NIH. This project was also funded in part by the grant Health Information Technology to Reduce Healthcare-Associated Infections (R01-NR010822, Elaine Larson).
Footnotes
Disclosures: The remaining authors have no conflicts of interest to disclose.
Author contributions:
Margot E. Cohen: Analysis and interpretation of data, drafting of manuscript
Joanne M. Hathway: Study concept and design, drafting of the manuscript
Hojjat Salmasian: Acquisition of data
Jianfang Liu: Clinical electronic data extraction and processing
Melissa Terry: Acquisition of data
Julian A. Abrams: Study concept and design, critical revision of manuscript
Daniel E. Freedberg: Study concept and design, analysis and interpretation of data, statistical analysis, drafting of manuscript
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Goto M, Al-Hasan MN. Overall burden of bloodstream infection and nosocomial bloodstream infection in North America and Europe. Clinical Microbiology and Infection. 2013;19:501–509. doi: 10.1111/1469-0691.12195. [DOI] [PubMed] [Google Scholar]
- 2.Prowle JR, Echeverri JE, Ligabo EV, et al. Acquired bloodstream infection in the intensive care unit: incidence and attributable mortality. Crit Care. 2011;15:R100. doi: 10.1186/cc10114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tabah A, Koulenti D, Laupland K, et al. Characteristics and determinants of outcome of hospital-acquired bloodstream infections in intensive care units: the EUROBACT International Cohort Study. Intensive Care Med. 2012;38:1930–45. doi: 10.1007/s00134-012-2695-9. [DOI] [PubMed] [Google Scholar]
- 4.Lim SJ, Choi JY, Lee SJ, et al. Intensive care unit-acquired blood stream infections: a 5-year retrospective analysis of a single tertiary care hospital in Korea. Infection. 2014;42:875–81. doi: 10.1007/s15010-014-0651-z. [DOI] [PubMed] [Google Scholar]
- 5.Pronovost P, Needham D, Berenholtz S, et al. An intervention to decrease catheter-related bloodstream infections in the ICU. N Engl J Med. 2006;355:2725–32. doi: 10.1056/NEJMoa061115. [DOI] [PubMed] [Google Scholar]
- 6.Zingg W, Imhof A, Maggiorini M, et al. Impact of a prevention strategy targeting hand hygiene and catheter care on the incidence of catheter-related bloodstream infections. Crit Care Med. 2009;37:2167–73. doi: 10.1097/CCM.0b013e3181a02d8f. quiz 2180. [DOI] [PubMed] [Google Scholar]
- 7.Johnson MT, Reichley R, Hoppe-Bauer J, et al. Impact of previous antibiotic therapy on outcome of Gram-negative severe sepsis. Crit Care Med. 2011;39:1859–65. doi: 10.1097/CCM.0b013e31821b85f4. [DOI] [PubMed] [Google Scholar]
- 8.Berenholtz SM, Pronovost PJ, Lipsett PA, et al. Eliminating catheter-related bloodstream infections in the intensive care unit. Crit Care Med. 2004;32:2014–20. doi: 10.1097/01.ccm.0000142399.70913.2f. [DOI] [PubMed] [Google Scholar]
- 9.Prevention CfDCa. Healthcare-associated infections prevalence survey [Google Scholar]
- 10.Barkun AN, Bardou M, Pham CQ, et al. Proton pump inhibitors vs. histamine 2 receptor antagonists for stress-related mucosal bleeding prophylaxis in critically ill patients: a meta-analysis. Am J Gastroenterol. 2012;107:507–20. doi: 10.1038/ajg.2011.474. quiz 521. [DOI] [PubMed] [Google Scholar]
- 11.ASHP Therapeutic Guidelines on Stress Ulcer Prophylaxis. ASHP Commission on Therapeutics and approved by the ASHP Board of Directors on November 14, 1998. Am J Health Syst Pharm. 1999;56:347–79. doi: 10.1093/ajhp/56.4.347. [DOI] [PubMed] [Google Scholar]
- 12.Krag M, Perner A, Wetterslev J, et al. Stress ulcer prophylaxis versus placebo or no prophylaxis in critically ill patients. A systematic review of randomised clinical trials with meta-analysis and trial sequential analysis. Intensive Care Med. 2014;40:11–22. doi: 10.1007/s00134-013-3125-3. [DOI] [PubMed] [Google Scholar]
- 13.O'Leary JG, Reddy KR, Wong F, et al. Long-term use of antibiotics and proton pump inhibitors predict development of infections in patients with cirrhosis. Clin Gastroenterol Hepatol. 2015;13:753–9. e1–2. doi: 10.1016/j.cgh.2014.07.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xu HB, Wang HD, Li CH, et al. Proton pump inhibitor use and risk of spontaneous bacterial peritonitis in cirrhotic patients: a systematic review and meta-analysis. Genet Mol Res. 2015;14:7490–501. doi: 10.4238/2015.July.3.25. [DOI] [PubMed] [Google Scholar]
- 15.Doig CJ, Sutherland LR, Sandham JD, et al. Increased intestinal permeability is associated with the development of multiple organ dysfunction syndrome in critically ill ICU patients. Am J Respir Crit Care Med. 1998;158:444–51. doi: 10.1164/ajrccm.158.2.9710092. [DOI] [PubMed] [Google Scholar]
- 16.Brenner DA, Paik YH, Schnabl B. Role of Gut Microbiota in Liver Disease. J Clin Gastroenterol. 2015;49(Suppl 1):S25–7. doi: 10.1097/MCG.0000000000000391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zaborin A, Smith D, Garfield K, et al. Membership and behavior of ultra-low-diversity pathogen communities present in the gut of humans during prolonged critical illness. MBio. 2014;5:e01361–14. doi: 10.1128/mBio.01361-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Apte M, Neidell M, Furuya EY, et al. Using Electronically Available Inpatient Hospital Data for Research. Clin Transl Sci. 2011;4:338–45. doi: 10.1111/j.1752-8062.2011.00353.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. Journal of Chronic Diseases. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 20.Prevention CfDCa. Multidrug-Resistant Organism & Clostridium difficile Infection MDRO/CDI) Module [Google Scholar]
- 21.Madigan MT. Brock biology of microorganisms. San Francisco: Benjamin Cummings; 2012. [Google Scholar]
- 22.Brown KA, Khanafer N, Daneman N, et al. Meta-analysis of antibiotics and the risk of community-associated Clostridium difficile infection. Antimicrob Agents Chemother. 2013;57:2326–32. doi: 10.1128/AAC.02176-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Acar J. Broad- and narrow-spectrum antibiotics: an unhelpful categorization. Clin Microbiol Infect. 1997;3:395–396. doi: 10.1111/j.1469-0691.1997.tb00274.x. [DOI] [PubMed] [Google Scholar]
- 24.Huang Y-q, Gou R, Diao Y-s, et al. Charlson comorbidity index helps predict the risk of mortality for patients with type 2 diabetic nephropathy. Journal of Zhejiang University Science B. 2014;15:58–66. doi: 10.1631/jzus.B1300109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Byung IkK, Hong Joo K, Jung Ho P, et al. Increased intestinal permeability as a predictor of bacterial infections in patients with decompensated liver cirrhosis and hemorrhage. Journal of Gastroenterology & Hepatology. 2011;26:550–557. doi: 10.1111/j.1440-1746.2010.06490.x. [DOI] [PubMed] [Google Scholar]
- 26.Ryan CM, Schmidt J, Lewandrowski K, et al. Gut macromolecular permeability in pancreatitis correlates with severity of disease in rats. Gastroenterology. 1993;104:890–5. doi: 10.1016/0016-5085(93)91027-f. [DOI] [PubMed] [Google Scholar]
- 27.Wallace JL, Syer S, Denou E, et al. Proton pump inhibitors exacerbate NSAID-induced small intestinal injury by inducing dysbiosis. Gastroenterology. 2011;141:1314–22. 1322.e1–5. doi: 10.1053/j.gastro.2011.06.075. [DOI] [PubMed] [Google Scholar]
- 28.Washio E, Esaki M, Maehata Y, et al. Proton Pump Inhibitors Increase Incidence of Nonsteroidal Anti-Inflammatory Drug-Induced Small Bowel Injury: A Randomized, Placebo-Controlled Trial. Clin Gastroenterol Hepatol. 2016;14:809–815. e1. doi: 10.1016/j.cgh.2015.10.022. [DOI] [PubMed] [Google Scholar]
- 29.Jones KR, Maddox CW, Ridgway MD, et al. Incidence of bacteremia following upper gastrointestinal endoscopy and biopsy in healthy dogs before, during, and after treatment with omeprazole. Am J Vet Res. 2013;74:239–42. doi: 10.2460/ajvr.74.2.239. [DOI] [PubMed] [Google Scholar]
- 30.Sommer F, Backhed F. The gut microbiota--masters of host development and physiology. Nat Rev Microbiol. 2013;11:227–38. doi: 10.1038/nrmicro2974. [DOI] [PubMed] [Google Scholar]
- 31.Freedberg DE, Toussaint NC, Chen SP, et al. Proton Pump Inhibitors Alter Specific Taxa in the Human Gastrointestinal Microbiome: A Crossover Trial. Gastroenterology. 2015;149:883–5. e9. doi: 10.1053/j.gastro.2015.06.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhernakova A, Kurilshikov A, Bonder MJ, et al. Population-based metagenomics analysis reveals markers for gut microbiome composition and diversity. Science. 2016;352:565–9. doi: 10.1126/science.aad3369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Williams C, McColl KE. Review article: proton pump inhibitors and bacterial overgrowth. Aliment Pharmacol Ther. 2006;23:3–10. doi: 10.1111/j.1365-2036.2006.02707.x. [DOI] [PubMed] [Google Scholar]
- 34.Verdu E, Viani F, Armstrong D, et al. Effect of omeprazole on intragastric bacterial counts, nitrates, nitrites, and N-nitroso compounds. Gut. 1994;35:455–60. doi: 10.1136/gut.35.4.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Faleck DM, Salmasian H, Furuya EY, et al. Proton Pump Inhibitors Do Not Increase Risk for Clostridium difficile Infection in the Intensive Care Unit. Am J Gastroenterol. 2016;111:1641–1648. doi: 10.1038/ajg.2016.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gocmez C, Celik F, Tekin R, et al. Evaluation of risk factors affecting hospital-acquired infections in the neurosurgery intensive care unit. Int J Neurosci. 2014;124:503–8. doi: 10.3109/00207454.2013.863773. [DOI] [PubMed] [Google Scholar]
- 37.Blaser MJ. Antibiotic use and its consequences for the normal microbiome. Science. 2016;352:544–5. doi: 10.1126/science.aad9358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Krag M, Perner A, Wetterslev J, et al. Prevalence and outcome of gastrointestinal bleeding and use of acid suppressants in acutely ill adult intensive care patients. Intensive Care Med. 2015;41:833–45. doi: 10.1007/s00134-015-3725-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.