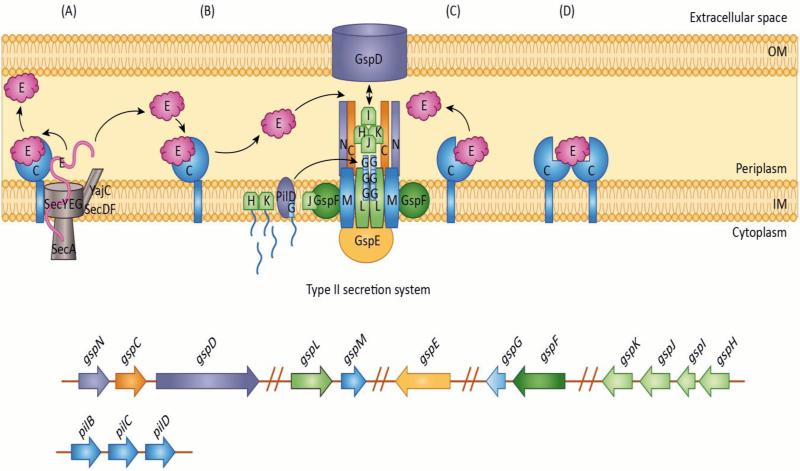
Figure 1. T2SS chaperone and effector interactions in A. nosocomialis M2.
In A. nosocomialis M2 the gsp genes required for T2SS are located in six separate operons. Secretion of type II effectors, CpaA and LipA, require specific membrane associated chaperones, CpaB and LipB. How these chaperones (C in figure) function in the secretion of their cognate effectors (E in figure) is unknown. This figure depicts potential chaperone-effector interaction mechanisms:
A) The effector is transported unfolded from the cytoplasm to the periplasmic space by the Sec secretion system. Once in the periplasm, the chaperone interacts with the effector initiating folding. The effector is then released into the periplasmic space, where it will eventually be secreted to the extracellular space by the T2SS. Alternatively, the effector may remain bound to the chaperone until it is released for secretion by the T2SS thereby preventing activity of the effector protein.
B) Upon entering the periplasmic space through the Sec secretion system, the effector folds. Once folded, it interacts with its chaperone. The interaction between the effector and chaperone may block activity of the effector protein through sequestration of the effector until it is released for secretion by the type II secretion system. C) The chaperone may function to guide the effector to the type II secretion machinery, through a secondary interaction with a component of the type II secretion system. D) The chaperone may function as a multimer when binding the effector protein.
OM: outer membrane, IM: Inner membrane.