Fig. 5. Effects of gabapentin on responses of vlPAGon neurons.
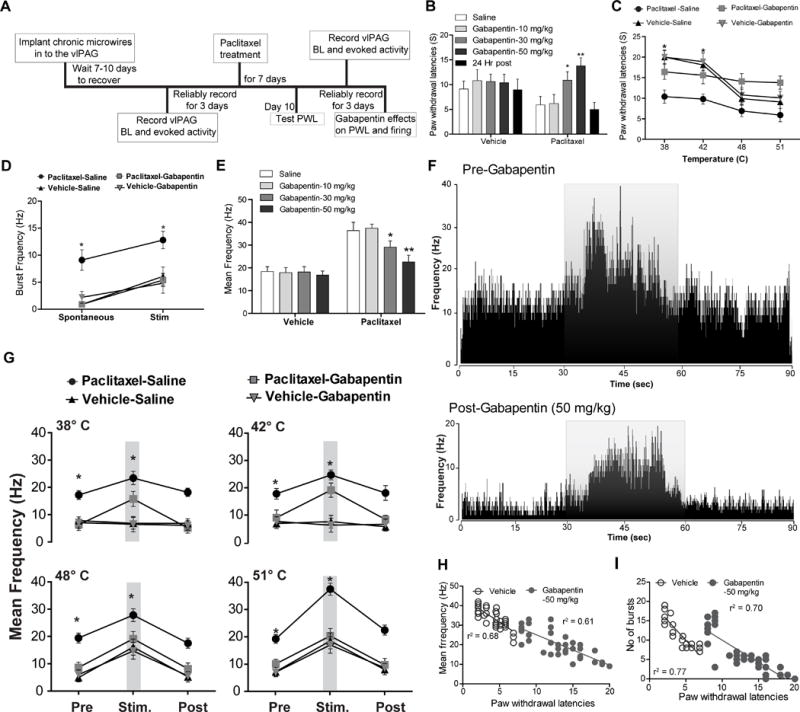
(A) Timeline of vlPAG recordings to evaluate effects of gabapentin in pre and post paclitaxel treated rats. (B) Systemic (intraperitoneal) administration of gabapentin resulted in dose-dependent antinociceptive effects on paw withdrawal latencies in paclitaxel treated rats but not in naïve (vehicle treated) rats. (C) Administration of gabapentin (50 mg/kg) reversed thermal hyperalgesia in paclitaxel treated rats but not in vehicle-treated rats. (D) Gabapentin administration (50 mg/kg) significantly reduced vlPAGon spontaneous and noxious thermal stimulus (51°C)-evoked burst neuronal firing in paclitaxel-treated rats but not in vehicle-treated rat. (E) Administration of gabapentin resulted in dose-dependent decrease in vlPAGon neuronal firing in paclitaxel treated rats but not in vehicle-treated rats. (F) Representative rate meter histograms show a significant reduction of spontaneous and noxious thermal stimulus (51°C) evoked activity in the vlPAGon neuron by gabapentin (50 mg/kg) i.p in the PAG neurons in paclitaxel-treated rats one hr after gabapentin treatment. The grey overlay represents the thermal stimulation. (G) Systemic administration of gabapentin (50 mg/kg) resulted in significant reduction in the vlPAGon neuronal firing evoked by the non-noxious thermal stimulus (38°C and 42°C) and noxious thermal stimulus (48°C and 51°C) in the paclitaxel-treated rats, but no effects were seen in vehicle-treated rats. (H) Gabapentin mediated attenuation of evoked neuronal firing (F(1, 37)= 56.44; p< 0.0001) and (I) burst firing (F(1,22)= 82.53; p< 0.0001) is directly correlated with elevated PWLs observed after gabapentin treatment in the paclitaxel-treated rats. * P < 0.05, ** P < 0.01; compared to pre-treatment levels. Two-way ANOVA followed by Bonferroni post hoc test. Each value is the mean ± SD; PWLs represent the mean of three trials; N=14 rats per group.
