Figure 6. BIM upregulation contributes to HDAC inhibitor-induced killing in malignant B-cell lines.
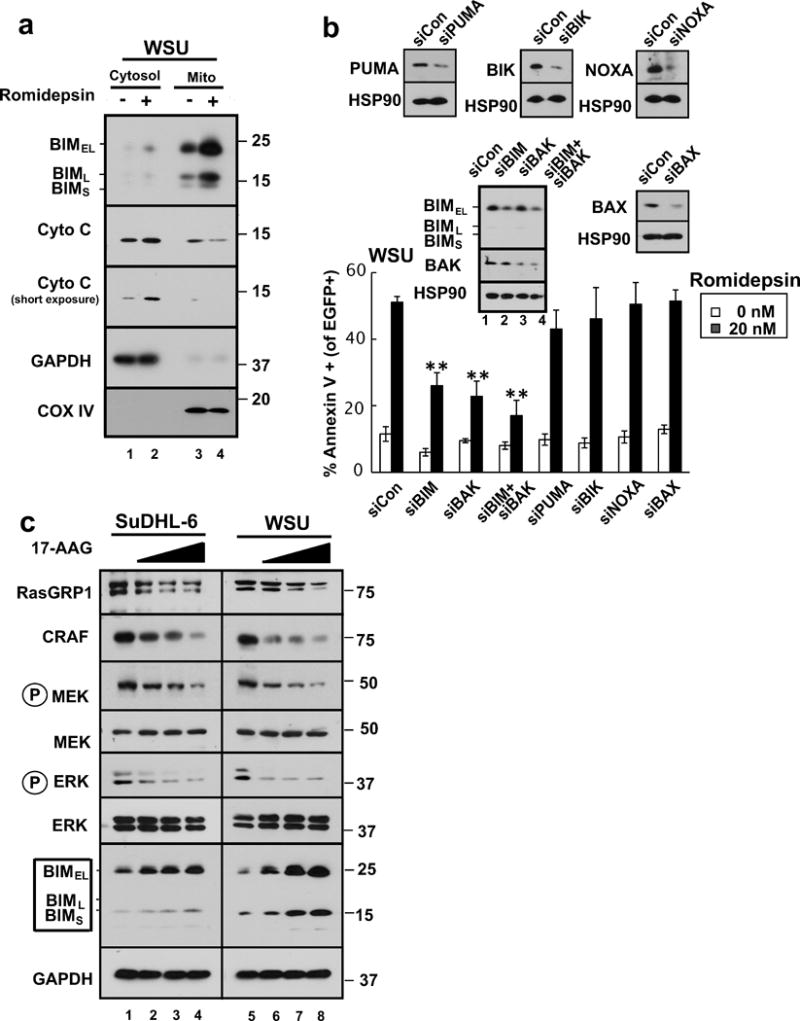
(a) After WSU cells were treated with diluent (0.1% DMSO, −) or 20 nM romidepsin (+) in the presence of 5 μM Q-VD-OPh for 24 h, the indicated subcellular fractions were isolated and subjected to immunoblotting. GAPDH served as a marker for cytosol and cytochrome c oxidase subunit IV (CoX IV) served as a marker for mitochondria. (b) 24 hours after transfection of the indicated siRNAs along with plasmid encoding EGFP-histone H2B (to mark successfully transfected cells), WSU cells were treated for 24 h with diluent or 20 nM romidepsin before staining with APC-conjugated annexin V and analysis by 2-color flow cytometry. Error bars, ± SD of three independent experiments. **, p <0.01. Inset: Immunoblots of whole cell lysates prepared from siRNA-treated cells incubated in drug-free medium in parallel with samples harvested for flow cytometry. HSP90 served as a loading control. (c) SuDHL-6 and WSU cells were treated for 48 h with diluent (lanes 1 and 5, respectively) or the HSP90 inhibitor 17AAG at 0.3, 1, or 3 μM (lanes 2–4 and 6–8, respectively) or diluent in the presence of 5 μM Q-VD-OPh. Whole cell lysates were subjected to SDS-PAGE and probed with the indicated antibodies. GAPDH served as a loading control.
