Abstract
Background
There remains uncertainty regarding whether a single fasting glucose measurement is sufficient to predict risk of adverse perinatal outcomes.
Methods
We included 12,594 pregnant women who underwent a 75-g oral glucose-tolerance test (OGTT) at 22–28 weeks' gestation in the Born in Guangzhou Cohort Study, China. Outcomes were large for gestational age (LGA) baby, cesarean section, and spontaneous preterm birth. We calculated the area under the receiver operator characteristic curves (AUCs) to assess the capacity of OGTT glucose values to predict adverse outcomes, and compared the AUCs of different components of OGTT.
Results
1325 women had a LGA baby (10.5%). Glucose measurements were linearly associated with LGA, with strongest associations for fasting glucose (odds ratio 1.37, 95% confidence interval 1.30–1.45). Weaker associations were observed for cesarean section and spontaneous preterm birth. Fasting glucose have a comparable discriminative power for prediction of LGA to the combination of fasting, 1 h, and 2 h glucose values during OGTT (AUCs, 0.611 vs. 0.614, P = 0.166). The LGA risk was consistently increased in women with abnormal fasting glucose (≥ 5.1 mmol/l), irrespective of 1 h or 2 h glucose levels.
Conclusions
A single fasting glucose measurement performs comparably to 75-g OGTT in predicting risk of having a LGA baby.
Keywords: Fasting plasma glucose, Oral glucose-tolerance test, Large for gestational age, Cesarean section, Spontaneous preterm birth
Highlights
-
•
Fasting glucose had a significantly stronger association with large for gestational age than post-load glucoses.
-
•
Single fasting glucose has comparable predictive ability of large for gestational age to 75g OGTT.
-
•
Single fasting glucose test can be an alternative for identifying high risk of large for gestational age in Chinese women.
The new criteria for gestational diabetes (GDM) screening and diagnosis suggests that single abnormal of a fasting, 1 h or 2 h glucose measured by an universal, single-stage screening of 2 h 75-g oral glucose-tolerance test (OGTT) is adequate to make a diagnosis. However, the utility of each glucose measurement in the prediction of poor perinatal outcomes was unclear. Here, we reported single fasting plasma glucose performs comparably to 75 g OGTT in identification of women at increased risk of delivering a large for gestational age baby. Single fasting glucose testing can be an alternative for GDM screening, which will substantially reduce the psychological impact, opportunity and direct health costs for women and their families.
1. Introduction
Large for gestational age (LGA), defined as a birth weight ≥ 90th percentile for gestational age, is the predominant adverse outcome associated with maternal hyperglycemia (Langer et al., 2005, Metzger et al., 2008). LGA is the main factor underlying birth trauma and preterm birth, as well as obstructed labor, that leads to cesarean delivery (Kc et al., 2015, Zhang et al., 2008). Long-term effects of LGA for the offspring include obesity, the metabolic syndrome, type 2 diabetes and insulin resistance (Damm et al., 2016).
In clinical practice, the main aim of gestational diabetes mellitus (GDM) treatment is to control glucose metabolism and thus reducing fetal macrosomia and obstetric complications as well as to prevent obesity in the offspring. Treatment of GDM is supposed to decrease the risk of fetal macrosomia (Crowther et al., 2005, Landon et al., 2009). In 2010, the International Association of the Diabetes and Pregnancy Study Groups (IADPSG) recommended new criteria for GDM diagnosis, based on odds ratios of abnormal birth weight, cord C-peptide and percent body fat observed in the Hyperglycemia and Adverse Pregnancy Outcome (HAPO) study (Metzger et al., 2008), with the primary aim of prevention of obesity risk among high-risk offspring. The test criteria include relatively lower cut-off values and single abnormal of a fasting, 1 h or 2 h glucose measured by an universal, single stage screening of 2 h 75-g oral glucose-tolerance test (OGTT) is adequate to make a diagnosis, which consequently increased the prevalence of GDM in many countries including China adopted this criteria (Cundy et al., 2014). Although OGTT has been well recognized as the “gold standard” for GDM diagnosis, it has many disadvantages of not reproducible, time-consuming and fairly demanding for both the pregnant women and the laboratory (Davidson, 2002, Hanna and Peters, 2002). Given the uncertainty of the utility of each glucose measurement in the prediction of fetal macrosomia and the significant resource implications of the IADPSG criteria, there remains controversy regarding this screening methodology for identification of macrosomia risk during pregnancy (Kalter-Leibovici et al., 2012).
In the setting of developing countries with limited resource, the screening and management of high-risk population may be more important and cost-effective than GDM diagnosis. Fasting plasma glucose (FPG) provides a cheap, acceptable reliable, reproducible alternative GDM screening method to the OGTT for the last three decades (Mortensen et al., 1985, Zhu et al., 2013), with renewed attention following introduction of the IADPSG criteria. Early studies suggested that FPG had significantly higher predictive value for LGA in comparison to post-load glucose, independent of maternal BMI and 2 h glucose value (Disse et al., 2013, Legardeur et al., 2014). A recent systematic review also found that fasting glucose concentration has stronger associations with LGA than post-load glucose concentration (Farrar et al., 2016). FPG performance has been largely determined by utility to GDM detection using specific criteria, with limited information regarding prediction of adverse pregnancy outcomes (Agarwal, 2016). However, the U.S. Preventive Services Task Force has suggested that the gold-standard for GDM screening tests, should include an acceptable, agreed set of relevant pregnancy outcomes (Donovan et al., 2013). There remains uncertainty regarding whether a single FPG measurement is sufficient for prediction of increased risk of adverse perinatal outcomes.
The aim of the study was to evaluate the performance of single FPG measurement versus a complete 75-g OGTT for screening women at increased risk of perinatal outcomes, primarily abnormal birth weight, in a large contemporary cohort of Chinese pregnant women.
2. Methods
2.1. Study Design and Participants
The Born in Guangzhou Cohort Study (BIGCS) is a prospective birth cohort study conducted in the Guangzhou Women and Children's Medical Center (GWCMC), China. This hospital serves women and children in Guangzhou city, including approximately 16,000 deliveries per year. We recruited pregnant women who (1) resided in Guangzhou, (2) intended to remain in Guangzhou with their child for at least 3 years, and (3) intended to receive routine antenatal care and deliver at the GWCMC. 15,198 women participated in BIGCS and delivered between February 2012 and June 2016 were eligible for the present analysis. We excluded those women with pre-pregnancy diabetes or chronic hypertension, multiple pregnancies, termination of pregnancy or missing OGTT or delivery data. Fig. 1 shows the participants' flowchart for this study. The women who met the criteria of diabetes mellitus in pregnancy (n = 70, 0.5%, i.e. fasting plasma glucose ≥ 7.0 mmol/l or 2 h 75 g post-load plasma glucose ≥ 11.1 mmol/l (WHO, 2013)) were included for analysis.
Fig. 1.
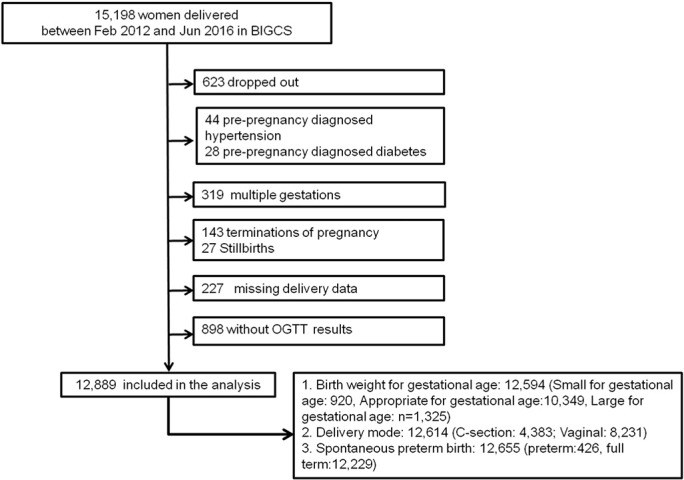
The recruitment and participation flowchart.
At recruitment, all eligible participants provided written informed consent, and completed a self-administered comprehensive questionnaire to obtain demographic, socio-economic, environmental, lifestyle, occupational and medical information. The study protocol was approved by GWCMC Ethics Approval Board.
2.2. Oral Glucose-Tolerance Test
Eligible participants underwent a standard 2 h 75 g OGTT between 22 and 28 weeks' gestation. Women were given instructions by doctors to follow the WHO procedures to fast overnight (8–14 h) before testing. Before drawing blood, the nurses confirmed with the pregnant women that they have an overnight fasting. 2 ml blood samples were collected at fasting, 1 h, and 2 h after the women receipt of 300 ml water in which 75 g of anhydrous glucose dissolved, respectively, using NaF/EDTA tubes. Blood samples were stored at room temperature before 3 time-points of glucose were all drew. Once the samples were sent to the laboratory, they were centrifuged and plasma glucose was measured immediately by a hexokinase method using Beckman Coulter AU5800 automatic analyzer (Beckman Coulter®, California, United States). The laboratory has achieved ISO15189 certification by China National Accreditation Service for Conformity Assessment. At each batch, quality control plasma was set to calculate the coefficients of variation. The coefficients of variation for low and high value were 1.63% and 1.43%, respectively. If values are outside 3SD, recalibration and retest were performed to confirm the result. The glucose concentration of OGTT and testing date were extracted from the GWCMC Laboratory Information System. Women whose prenatal 75-g OGTT results met or exceeded at least one threshold of the IADPSG criteria (FPG ≥ 5.1 mmol/l, 1 h glucose ≥ 10.0 mmol/l, and 2 h glucose ≥ 8.5 mmol/l) including those have overt diabetes in pregnancy received routine clinical intervention by diet and exercise therapy. After diet control for 3 to 5 days, the women with GDM were asked to self-monitored and recorded preprandrial blood glucose (30 min before breakfast/lunch/dinner), postprandial blood glucose (30 min after breakfast/lunch/dinner), and nocturnal blood glucose at home every day. Of these women, those with FPG ≥ 5.3 mmol/l or 2 h postprandial glucose was ≥ 6.7 mmol/l after diet therapy were prescribed insulin or glyburide in addition to diet and exercise (Obstetrics Subgroup and Group of Pregnancy with Diabetes Mellitus, 2014). Obstetrical complications such as pregnancy hypertension disease, hydramnios, and infection were monitored by the obstetricians.
2.3. Perinatal Outcomes
The main outcomes included birthweight (continuous), birthweight Z score (continuous), LGA, cesarean section (C-section), and spontaneous preterm birth (SPTB). Birthweight and other information, including gestational age at delivery, mode of delivery, newborn gender, and pregnancy complications were obtained from medical records using the hospital-based information system. Birth weight was measured by GWCMC midwives immediately after delivery. Birthweight Z scores were calculated using a local population-based birth weight reference (He et al., 2014). LGA was defined as a birthweight larger than the 90th percentile for gestational age by gender, based on the same birth weight reference (He et al., 2014). Gestational age was estimated from ultrasound examination during the first- or second-trimester. SPTB was defined as birth following spontaneous preterm labor and/or preterm premature rupture of the membranes at < 37 weeks gestation, irrespective of mode of delivery (vaginal, cesarean section) obtained from medical records and independently confirmed by two pediatricians.
2.4. Statistical Analysis
Descriptive statistics were reported for continuous (mean, SD) and categorical (number, percentage) variables respectively. To examine associations between maternal glycemia and perinatal outcomes, each glucose measurement was considered as a continuous variable. Multiple linear and logistic regressions were used to evaluate continuous (birth weight, birth weight Z score) and categorical measures (LGA, C-section, and SPTB), respectively. Odds ratios (ORs) and regression coefficients were calculated for a 1-SD increase in each fasting, 1 h, and 2 h glucose measurement. All models were adjusted for maternal age, maternal education, maternal income, maternal pre-pregnancy body mass index (BMI), height, parity, maternal smoking or passive smoke exposure during pregnancy, family history of diabetes, use of assisted reproduction technology, baby gender and gestational age at OGTT date. To explore differences in associations in diverse social economic status, we did stratified analysis by maternal income and education as well as included an interaction term between maternal income or education and glucose measurement in the model.
We used receiver operating characteristic (ROC) analyses to assess the predictive capacity of FPG, 1 h, 2 h glucose for the categorical perinatal outcomes specified. To explore additional predictive power of post-load glucose values, we added 1 h and/or 2 h glucose values to the FPG model, calculating the respective area under the curve (AUC) measures. The areas under correlated ROC curves of different glucose measurements were compared using a non-parameteric approach developed by DeLong (Delong et al., 1988).
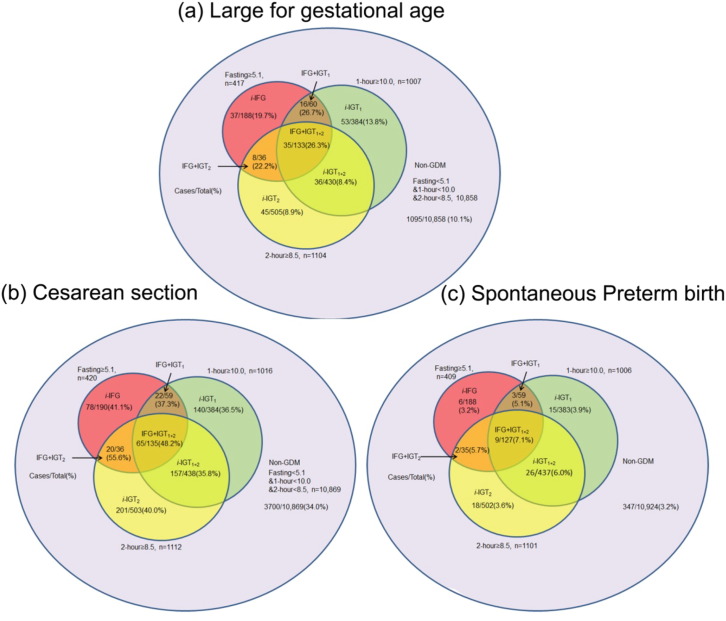
To explore the influence of gestational week of undertaking OGTT, we undertook a sensitivity analysis by repeating analyses restricted to women who underwent OGTT between 24 and 28 weeks' gestation. To evaluate the treatment effect, we performed a sensitivity analysis restricted to women without GDM, who were untreated, and we also conducted an analysis that added the GDM status as a proxy of use of treatment (yes or no) in the model for adjustment. To explore the risk of perinatal outcomes identified by each abnormal glucose measurement based on IADPSG criteria, we categorized women into eight mutually exclusive categories according to 2 h 75-g OGTT result (Fig. 3). The prevalence of perinatal outcomes in each group and the adjusted ORs were calculated, using no-GDM as the reference group.
Fig. 3.
Prevalence of perinatal outcomes by IADPSG criteria (glucose level).
‘Case’ indicates the number of outcome cases in corresponding category of glucose levels. ‘Total’ indicates the total number of pregnant women in corresponding category of glucose levels. ‘%’ indicates the prevalence of outcomes in corresponding category of glucose levels. Non-GDM: no glucose impairment; i-IFG: isolated impaired fasting glucose if FPG ≥ 5.1 mmol/l and both 1 h glucose < 10.0 mmol/l and 2 h glucose < 8.5 mmol/l; i-IGT1: single isolated impaired 1 h glucose tolerance if 1 h glucose ≥ 10.0 mmol/l and both 2 h glucose < 8.5 mmol/l and FPG < 5.1 mmol/l; i-IGT2: single isolated impaired 2 h glucose tolerance if 2 h glucose ≥ 8.5 mmol/l and both 1 h glucose < 10.0 mmol/l and FPG < 5.1 mmol/l; i-IGT1 + 2: double-isolated impaired glucose tolerance if both 1 h glucose ≥ 10.0 mmol/l and 2 h glucose ≥ 8.5 mmol/l but FPG < 5.1 mmol/l; IFG + IGT1: combined IFG and IGT1 if FPG ≥ 5.1 mmol/l and 1 h glucose ≥ 10.0 mmol/l but 2 h glucose < 8.5 mmol/l; IFG + IGT2: combined IFG and IGT2 if FPG ≥ 5.1 mmol/l and 2 h glucose ≥ 8.5 mmol/l but 1 h glucose < 10.0 mmol/l; IFG + IGT1 + 2: combined IFG and IGT1 + 2 if FPG ≥ 5.1 mmol/l and 1 h glucose ≥ 10.0 mmol/l and 2 h ≥ 8.5 mmol/l.
All analyses were performed using SAS 9.2 software (SAS Institute, Cary, NC, USA). A P value < 0.05 was considered to indicate statistical significance.
3. Results
A total of 15,198 pregnant women recruited to the cohort study delivered between Feb 2012 and Jun 2016 (Fig. 1). We excluded 2309 women for the following reasons: withdrawal (n = 623), diagnosis of pre-pregnancy hypertension (n = 44), diabetes (n = 28), multiple pregnancy (n = 319), termination of pregnancy (n = 143), stillbirth (n = 27), missing delivery data (n = 227), and missing OGTT (n = 898). We included information obtained from 12,889 mother-newborn pairs in the analyses. There were no significant differences in birthweight or gestational age between those women included in the final analysis (n = 12,889) and those who had missing OGTT results (n = 898). The characteristics between women who dropped out or missing OGTT results and delivery data and women who were included in the analysis were only statistical different in parity (Appendix Table 1).
Participant baseline characteristics and outcome measures are summarized in Tables 1 and 2. The mean FPG, 1 h, and 2 h plasma glucose levels were 4.3 mmol/l, 7.7 mmol/l, and 6.6 mmol/l, respectively. Using the IADPSG criteria, there were 1779 (N = 12,889, 13.8%) women diagnosed with GDM who received a subsequent diet and exercise advice (425 with FPG ≥ 5.1 mmol/l, 1354 with FPG < 5.1 mmol/l and abnormal post-load glucose). Eleven (11/12,889, 0.1%) women went on to receive insulin therapy. The overall prevalence of LGA, C-section, and SPTB was 10.5% (1325/12,594), 34.8% (4383/12,614), and 3.4% (426/12,655), respectively (Table 2).
Table 1.
Study participant characteristics (n = 12,889).
| Characteristics | No. of total (%) | Mean ± SD |
|---|---|---|
| Maternal characteristic | ||
| Age, years | 12,889 (100.0) | 29.1 ± 3.4 |
| Plasma glucose, mmol/l | ||
| Fasting | 12,889 (100.0) | 4.3 ± 0.4 |
| 1 h | 12,889 (100.0) | 7.7 ± 1.7 |
| 2 h | 12,889 (100.0) | 6.6 ± 1.4 |
| Gestation week at time of OGTT, week | 12,889 (100.0) | 24.5 ± 1.6 |
| Income (RMB), % | ||
| < 1500 | 1204 (9.8) | |
| 1500–4500 | 3647 (29.7) | |
| 4501–9000 | 5248 (42.7) | |
| ≥ 9001 | 2179 (17.8) | |
| Education, % | ||
| Middle school or below | 1205 (9.4) | |
| College | 3230 (25.1) | |
| Undergraduate | 6864 (53.3) | |
| Postgraduate | 1590 (12.3) | |
| Pre-pregnancy body mass index, kg/m2 | 12,636 (98.0) | 20.4 ± 2.7 |
| Height, cm | 12,751 (98.9) | 160.0 ± 4.9 |
| Family history of diabetes | 1273 (10.2) | |
| Smoking during pregnancy, % | 62 (0.5) | |
| Passive smoking during pregnancy, % | 3695 (29.5) | |
| Parity at enrollment ≥ 1, % | 1768 (13.9) | |
| Use of assisted reproduction technology, % | 421 (3.4) | |
| Newborn characteristic | ||
| Gender | ||
| Boy, % | 6683 (52.5) | |
| Girl, % | 6039 (47.5) |
SD = standard deviation; OGTT = oral glucose-tolerance test.
Table 2.
The prevalence of perinatal outcomes (n = 12,889).
| Characteristic or outcome | No. of participants (%) | Mean ± SD |
|---|---|---|
| Obstetrical outcomes | ||
| Cesarean delivery, % | 4383 (34.8) | |
| Newborn outcomes | ||
| Gestational age at delivery, week | 12,888 (100.0) | 38.8 ± 1.5 |
| Preterm birth, % | 630 (4.9) | |
| Spontaneous preterm birth, % | 426 (3.4) | |
| Birth weight, g | 12,678 (98.4) | 3191.6 ± 425.6 |
| Birth weight Z score | 12,678 (98.4) | 0.1 ± 1.0 |
| Birth weight for gestational age, % | ||
| SGA | 920 (7.3) | |
| AGA | 10,349 (82.2) | |
| LGA | 1325 (10.5) |
SD = standard deviation; AGA = appropriate for gestational age was defined as 10th ≤ birth weight ≤ 90th percentile for gestational age; SGA = small for gestational age was defined as birth weight < 10th percentile for gestational age; LGA = large for gestational age was defined as birth weight > 90th percentile for gestational age on the basis of local population-based birth weight reference.
Table 3 shows the association between maternal glucose and perinatal outcomes. For birthweight and birthweight Z score, the coefficients for 1-SD increase in all OGTT measures glucose were significant. Among the categorical outcomes, the associations were strongest for LGA (ORs for each 1-SD increase in glucose level range, 1.17 to 1.37). For cesarean section and spontaneous preterm birth, the associations were weaker. The associations between fasting glucose and birthweight, birthweight Z score, and LGA were strongest for fasting glucose as compared to both post-load glucose concentrations. No statistical evidence of an interaction of glucose measurements (fasting, 1 h, and 2 h) with maternal income and education was found (Appendix Tables 2 and 3). Results of analyses restricted to women underwent OGTT at 24–28 gestation's weeks did not change significantly (Appendix Table 4). When we restricted to the women without a GDM diagnosis, we found that the association between fasting glucose and birthweight or LGA is still stronger than post-load glucose in non-GDM women (Appendix Table 5). When we used the GDM status as a proxy of use of treatment (yes or no) and included in model for adjustment, the results were similar to findings when the analysis was restricted to women without GDM (Appendix Table 6).
Table 3.
Adjusted coefficients or ORs for associations between maternal glucose (continuous variables) and perinatal outcomes.
| Outcomes | Total no. (no. with outcome) | Plasma glucose level |
||
|---|---|---|---|---|
| Fasting | At 1 h | At 2 h | ||
| Adjusted coefficients or ORs (95% CI)a |
Adjusted coefficients or ORs (95% CI)a |
Adjusted coefficients or ORs (95% CI)a | ||
| Entire cohort | ||||
| Birthweight, coefficients | 12,678 | 42.01 (34.55, 49.48) | 18.95 (11.38, 26.52) | 13.32 (5.71, 20.94) |
| Birthweight Z score, coefficients | 12,678 | 0.12 (0.10, 0.14) | 0.08 (0.06, 0.10) | 0.07 (0.05, 0.08) |
| Large for gestational age, ORs | 12,594 (1325) | 1.37 (1.30, 1.45) | 1.24 (1.16, 1.31) | 1.17 (1.11, 1.25) |
| Cesarean section, ORs | 12,614 (4383) | 1.12 (1.08, 1.17) | 1.03 (0.99, 1.07) | 1.04 (1.00, 1.09) |
| Spontaneous preterm birth, ORs | 12,655 (426) | 1.05 (0.95, 1.16) | 1.20 (1.09, 1.33) | 1.21 (1.10, 1.33) |
Abbreviations: CI, confidence interval, ORs, odd ratios.
Regression coefficients or ORs were for an increase in the glucose level of 1 SD (0.4 mmol/l for the fasting glucose level, 1.7 mmol/l for the 1 h glucose level, and 1.4 mmol/l for the 2 h glucose level). Adjusted for maternal age, income, educational level, smoking during pregnancy, second hand smoking exposure during pregnancy, pre-pregnancy BMI, height, family history of diabetes, gender, parity, assisted reproductive technology, gestational week at the OGTT.
The ROC curves evaluating the performance of OGTT glucose measurements for predicting perinatal outcomes are shown in Fig. 2. For LGA, the AUC of FPG was significantly higher than 1 h (0.611 vs. 0.566, P < 0.0001) and 2 h (0.611 vs. 0.551, P < 0.0001) glucose measurements and did not significantly increase after adding 1 h and 2 h measurements to the FPG predictive model. The AUCs for other two outcomes were all smaller than LGA. Similar results were found when the analysis restricted to women underwent OGTT at 24–28 gestation's weeks (Appendix Fig. 1) and women without GDM (Appendix Fig. 2). Although the P value for the comparison of FPG with OGTT measurements in women without GDM is < 0.05, the confidence intervals was rather close and the significance is more likely to be caused by large sample size.
Fig. 2.
Receiver operating characteristic curves (ROCs) of fasting, 1 h and 2 h glucose measurements for prediction of perinatal outcomes.
*The AUCs for 1 h or/and 2 h glucose were significantly different from fasting glucose (P < 0.05).
To assess the contribution of each glucose measure in identifying risk of outcomes, we classified participants into eight categories according to IADPSG diagnostic criteria. Fig. 3 and Appendix Table 7 showed the respective GDM prevalence and adjusted OR (used no-GDM as reference) for outcome measures by diagnostic category. LGA prevalence was significantly higher among women with abnormal FPG (≥ 5.1 mmol), irrespective of 1 h or 2 h post-load glucose levels (prevalence range 19.68%–26.67%, ORs range 1.72–2.62 in women with i-IFG, IFG + IGT1, IFG + IGT2, or IFG + IGT1 + 2). Among women with FPG < 5.1 mmol, the prevalence of LGA was relatively low, even for those abnormal 1 h or/and 2 h glucose level (prevalence 10.1%,13.8%, 8.9%, and 8.4% for no-GDM, i-IGT1, i-IGT2, i-IGT1 + 2 group; ORs [95%CI] were 1.27[0.92, 1.75] for i-IGT1, 0.87[0.62,1.20] for i-IGT2 and 0.77[0.53,1.11] for i-IGT1 + 2). Similar results were observed for cesarean section although there was no significant association for i-IFG, IFG + IGT1, IFG + IGT2, or IFG + IGT1 + 2. Women with two abnormal glucose measures seem to have a higher risk of SPTB.
4. Discussion
In this large-scale prospective cohort study, we observed continuous associations of fasting, 1 h and 2 h post-load glucose with LGA. Weaker associations were observed for cesarean section and spontaneous preterm birth. FPG have a comparable discriminative power for prediction of LGA to the combination of fasting, 1 h, and 2 h glucose values during OGTT. These findings are broadly consistent with those reported in a comparable Asian cohort study and recent systematic review (Aris et al., 2014, Farrar et al., 2016).
Numerous studies examining the FPG performance for screening GDM included the patients selected by clinical history or positive glucose challenge test, which was a biased population leading to improvement of FPG performance and limitation of the generation to entire population (Perucchini et al., 1999). The previous studies that explored FPG performance used GDM diagnosed by specific criteria as the gold standard rather than used pregnancy outcomes (Agarwal et al., 2011, Poomalar and Rangaswamy, 2013). The few studies comparing FPG performance to post-load glucose based on pregnancy outcomes reported comparable AUCs for post-load glucose in prediction of LGA to our study (0.578 for 1 h, 0.573 for 2 h in Trujillo et al.'s study (Trujillo et al., 2015); 0.549 for 2 h in Disse et al.'s study (Disse et al., 2013)). The AUCs for fasting glucose in prediction of LGA in present study was higher than that reported by Trujillo et al. (n = 4077, AUC: 0.553) and lower than that reported by Disse et al. (n = 75, AUC: 0.783). In line with our results, Disse et al. found that FPG was the only glucose value significantly predictive of LGA babies (Disse et al., 2013). In a sub-cohort of HAPO study, the Kalter-Leibovici et al. found that one-third of IADPSG-positive women had low risk of fetal macrosomia and they developed an alternative screening method largely by use of FPG ≥ 89 mg/dl (Kalter-Leibovici et al., 2012).
The lack of additional predictive power provided by inclusion of post-load glucoses in the FPG predictive model for LGA suggests that FPG alone could provide adequate diagnostic utility to identify those pregnant women with a higher risk of LGA. In the HAPO study women with IADPSG-positive FPG had the highest prevalenc of LGA (19.5%), compared to those with IADPSG-positive 1- and/or 2 h glucose level and normal FPG (12.6%) and IADPSG-negative women (8.2%) (Coustan et al., 2010). The result partially supports our finding that isolated abnormal 1 h and/or 2 h glucose have lower risk of LGA than abnormal FPG. Based on these findings, we suggest that screening for GDM in the purpose of screening and management of women at high risk of delivering an LGA baby can use a single FPG measurement instead of a universal one step of OGTT, especially in the areas with limited health resource. It is well established that FPG and post-load glucose levels reflect metabolic alterations among women with GDM and this relationship will not be observed from fasting glucose values in isolation. However, the aim of universal GDM screening is to inform risk stratification for LGA and fasting glucose measurements provides improved acceptability and cost-effectiveness.
Insulin has been proven to be the most important growth hormone (Eslamian et al., 2013) that promotes maternal hypertriglyceridemia during pregnancy (Butte, 2000), alters lipid transport to the fetus (Radaelli et al., 2009) and up-regulates other placental nutrient transport pathways (Brett et al., 2014). Previous studies revealed that basal insulin resistance (usually with higher level of insulin) plays the most crucial role on elevation of FPG level, whereas the decrease in glucose-induced insulin secretion (insulin decreased) is the most important factor contributing to elevation of 2 h glucose level in the general population (Hanefeld et al., 2003). Some authors also reported that women with elevated FPG during pregnancy have higher fasting insulin levels and primarily hepatic insulin resistance and are more likely to require insulin therapy than those with isolated elevated post-load glucose levels (Disse et al., 2013, Zamzami, 2007). These studies suggest that elevated FPG exerts a greater effect on fetal growth through increase of maternal insulin level than elevated post-load glucose levels.
The study had several strengths; firstly, most previous studies that have explored the performance of FPG for screening GDM were based on various criteria for GDM diagnosis as opposed to pregnancy outcomes (Agarwal, 2016). We conducted a large-scale prospective cohort study to assess the utility of a single FPG screening test as an alternative method for GDM diagnosis, targeted at identification of increased LGA risk. Secondly, our study provides high quality, accurate information on outcome measures, reducing misclassification and measurement error. Thirdly, we adjusted for a comprehensive range of potential confounding factors in our analyses, allowing us to assess the independent effect of maternal hyperglycemia exposure from co-existing maternal and fetal exposures. In the present study, we did not exclude the women who met the criteria of diabetes in pregnancy because one of the aims was to analyse the association of maternal hyperglycemia in pregnancy with adverse pregnancy outcomes as well as the clinical managements for diabetes mellitus in pregnancy and gestational diabetes was similar in China (Obstetrics Subgroup and Group of Pregnancy with Diabetes Mellitus, 2014). Finally, the doctors gave the women an instruction for OGTT to follow the standard procedures recommended by WHO and the nurses who drew the blood confirmed the overnight fasting of the pregnant women before OGTT, which largely reduced the inadequate fasting.
Our study had some limitations. Firstly, we did not collect cord-blood C-peptide concentrations and newborn fat mass, which had been used to develop IADPSG criteria. In human studies, strong associations of LGA and neonatal adiposity with fetal hyperinsulinemia were observed, independent of confounding factors (Langer et al., 2005). Although increased neonatal adiposity is one of the indicators of abnormal fetal development in intrauterine exposure to maternal hyperglycaemia, long-term effects of neonatal adiposity remain undetermined (Logan et al., 2016). Moreover, we considered that the other two surrogate markers were of less relevance for clinical practice. The consistency in observed association between FPG and both outcome measures to those reported in previous studies indicate the relevance of our endpoints to be comparable to those obtained in the HAPO study (Metzger et al., 2008). Secondly, in our study, women with GDM diagnosis routinely receive diet and exercise intervention, with only a small proportion of women received insulin therapy. Two randomized control trials (RCTs) have indicated that treatment of mild GDM could reduce birth weight (Crowther et al., 2005, Landon et al., 2009). Treatment of GDM may be expected to lower the prevalence of adverse outcomes and the natural effect of maternal hyperglycemia on fetal growth. Whereas, the sensitivity ROC analysis in women without GDM, who were untreated, suggests that single FPG still have comparable discriminative power for predicting LGA in untreated women in Chinese population The sensitivity analysis adjusting for GDM treatment also showed similar associations of OGTT measurements with LGA to those in non-GDM women. Admittedly, further studies are needed to determine the optimal threshold of FPG for high risk screening. Thirdly, BIGCS participants were recruited at a tertiary care centre and are likely to have higher socio-economic status which may limit generalisability of our findings to other settings. We did not however observe any significant interactions between maternal income or educational level and glucose measures on LGA, suggesting the effects of glucose levels were similar across populations with different socio-economic status. In the present study, about 12% of participants were lost to follow-up or had missing data. We found baseline characteristics of these women and women who were included in the analysis not to be statistical different except for parity. Exclusion of these women would not change the results significantly.
To conclude, we report that 1 h and/or 2 h plasma glucose levels contribute little to LGA prediction in the present analyses. We propose that a single FPG screening can provide a valid measurement to identify higher risk of LGA in Chinese population. It will markedly reduce laboratory burden, numbers of intervention, rates of early-term birth and C-section, and neonates requiring special care, especially in China where medical resources were limited in many areas. When generalizing our findings to other settings, the difference (or overlap) in distribution of abnormal fasting, 1 h or 2 h between populations should be considered.
Funding Sources
This study is supported by the Guangzhou Science and Technology Bureau, Guangzhou, China (2011Y2-00025, 201508030037).
Conflicts of Interest
The authors declare no actual or potential competing financial interests.
Author Contributions
HX, XQ and KKC designed the birth cohort on which this study was based. XQ and HX designed the study and directed its implementation. SS, JL, JH, WL, NC, WX, MY, and LQ were involved in study design, questionnaires development, data collection and follow-up of participants. SS and LZ analysed the data and drafted the manuscript. JL and WX managed the data and did the statistical analysis. XQ, BWM, HX, and KC revised the manuscript. XQ and HX interpreted the data. All authors critically revised the manuscript and approved the final version.
Acknowledgments
We thank all the pregnant women who participated in the Born in Guangzhou Cohort Study participants and all staff in the cohort team for their contribution to this study, particularly the research nurses and midwives and other recruiting staff for their excellent work. We thank Dr. Suzanne Bartington for helping the language editing of the manuscript. The funding bodies had no role in study design, data collection or analysis, decision to publish, or preparation of the manuscript. The corresponding author had access to all data and made the final decision to submit for publication.
Footnotes
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.ebiom.2017.01.025.
Contributor Information
Huimin Xia, Email: huimin.xia876001@gmail.com.
Xiu Qiu, Email: qxiu0161@163.com.
Appendix A. Supplementary data
Supplementary material
References
- Agarwal M.M. Gestational diabetes mellitus: screening with fasting plasma glucose. World J. Diabetes. 2016;7:279–289. doi: 10.4239/wjd.v7.i14.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agarwal M.M., Weigl B., Hod M. Gestational diabetes screening: the low-cost algorithm. Int. J. Gynaecol. Obstet. 2011;115(Suppl. 1):S30–S33. doi: 10.1016/S0020-7292(11)60009-X. [DOI] [PubMed] [Google Scholar]
- Aris I.M., Soh S.E., Tint M.T., Liang S., Chinnadurai A., Saw S.M., Rajadurai V.S., Kwek K., Meaney M.J., Godfrey K.M., Gluckman P.D., Yap F.K., Chong Y.S., Lee Y.S. Effect of maternal glycemia on neonatal adiposity in a multiethnic Asian birth cohort. J. Clin. Endocrinol. Metab. 2014;99:240–247. doi: 10.1210/jc.2013-2738. [DOI] [PubMed] [Google Scholar]
- Brett K.E., Ferraro Z.M., Yockell-Lelievre J., Gruslin A., Adamo K.B. Maternal-fetal nutrient transport in pregnancy pathologies: the role of the placenta. Int. J. Mol. Sci. 2014;15:16153–16185. doi: 10.3390/ijms150916153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butte N.F. Carbohydrate and lipid metabolism in pregnancy: normal compared with gestational diabetes mellitus. Am. J. Clin. Nutr. 2000;71:1256S–1261S. doi: 10.1093/ajcn/71.5.1256s. [DOI] [PubMed] [Google Scholar]
- Coustan D.R., Lowe L.P., Metzger B.E., Dyer A.R. The Hyperglycemia and Adverse Pregnancy Outcome (HAPO) study: paving the way for new diagnostic criteria for gestational diabetes mellitus. Am. J. Obstet. Gynecol. 2010;202(654):e1–e6. doi: 10.1016/j.ajog.2010.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowther C.A., Hiller J.E., Moss J.R., Mcphee A.J., Jeffries W.S., Robinson J.S. Effect of treatment of gestational diabetes mellitus on pregnancy outcomes. N. Engl. J. Med. 2005;352:2477–2486. doi: 10.1056/NEJMoa042973. [DOI] [PubMed] [Google Scholar]
- Cundy T., Ackermann E., Ryan E.A. Gestational diabetes: new criteria may triple the prevalence but effect on outcomes is unclear. BMJ. 2014;348:g1567. doi: 10.1136/bmj.g1567. [DOI] [PubMed] [Google Scholar]
- Damm P., Houshmand-Oeregaard A., Kelstrup L., Lauenborg J., Mathiesen E.R., Clausen T.D. Gestational diabetes mellitus and long-term consequences for mother and offspring: a view from Denmark. Diabetologia. 2016;59:1396–1399. doi: 10.1007/s00125-016-3985-5. [DOI] [PubMed] [Google Scholar]
- Davidson M.B. Counterpoint: the oral glucose tolerance test is superfluous. Diabetes Care. 2002;25:1883–1885. doi: 10.2337/diacare.25.10.1883. [DOI] [PubMed] [Google Scholar]
- Delong E.R., Delong D.M., Clarke-Pearson D.L. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44:837–845. [PubMed] [Google Scholar]
- Disse E., Graeppi-Dulac J., Joncour-Mills G., Dupuis O., Thivolet C. Heterogeneity of pregnancy outcomes and risk of LGA neonates in Caucasian females according to IADPSG criteria for gestational diabetes mellitus. Diabete Metab. 2013;39:132–138. doi: 10.1016/j.diabet.2012.09.006. [DOI] [PubMed] [Google Scholar]
- Donovan L., Hartling L., Muise M., Guthrie A., Vandermeer B., Dryden D.M. Screening tests for gestational diabetes: a systematic review for the U.S. Preventive Services Task Force. Ann. Intern. Med. 2013;159:115–122. doi: 10.7326/0003-4819-159-2-201307160-00657. [DOI] [PubMed] [Google Scholar]
- Eslamian L., Akbari S., Marsoosi V., Jamal A. Effect of different maternal metabolic characteristics on fetal growth in women with gestational diabetes mellitus. Iran J. Reprod. Med. 2013;11:325–334. [PMC free article] [PubMed] [Google Scholar]
- Farrar D., Simmonds M., Bryant M., Sheldon T.A., Tuffnell D., Golder S., Dunne F., Lawlor D.A. Hyperglycaemia and risk of adverse perinatal outcomes: systematic review and meta-analysis. BMJ. 2016;354:i4694. doi: 10.1136/bmj.i4694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanefeld M., Koehler C., Fuecker K., Henkel E., Schaper F., Temelkova-Kurktschiev T. Insulin secretion and insulin sensitivity pattern is different in isolated impaired glucose tolerance and impaired fasting glucose: the risk factor in impaired glucose tolerance for atherosclerosis and diabetes study. Diabetes Care. 2003;26:868–874. doi: 10.2337/diacare.26.3.868. [DOI] [PubMed] [Google Scholar]
- Hanna F.W., Peters J.R. Screening for gestational diabetes; past, present and future. Diabet. Med. 2002;19:351–358. doi: 10.1046/j.1464-5491.2002.00684.x. [DOI] [PubMed] [Google Scholar]
- He J.R., Xia H.M., Liu Y., Xia X.Y., Mo W.J., Wang P., Cheng K.K., Leung G.M., Feng Q., Schooling C.M., Qiu X. A new birthweight reference in Guangzhou, southern China, and its comparison with the global reference. Arch. Dis. Child. 2014;99:1091–1097. doi: 10.1136/archdischild-2013-305923. [DOI] [PubMed] [Google Scholar]
- Kalter-Leibovici O., Freedman L.S., Olmer L., Liebermann N., Heymann A., Tal O., Lerner-Geva L., Melamed N., Hod M. Screening and diagnosis of gestational diabetes mellitus: critical appraisal of the new International Association of Diabetes in Pregnancy Study Group recommendations on a national level. Diabetes Care. 2012;35:1894–1896. doi: 10.2337/dc12-0041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kc K., Shakya S., Zhang H. Gestational diabetes mellitus and macrosomia: a literature review. Ann. Nutr. Metab. 2015;66(Suppl. 2):14–20. doi: 10.1159/000371628. [DOI] [PubMed] [Google Scholar]
- Landon M.B., Spong C.Y., Thom E., Carpenter M.W., Ramin S.M., Casey B., Wapner R.J., Varner M.W., Rouse D.J., Thorp J.M., Jr., Sciscione A., Catalano P., Harper M., Saade G., Lain K.Y., Sorokin Y., Peaceman A.M., Tolosa J.E., Anderson G.B. A multicenter, randomized trial of treatment for mild gestational diabetes. N. Engl. J. Med. 2009;361:1339–1348. doi: 10.1056/NEJMoa0902430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langer O., Yogev Y., Most O., Xenakis E.M. Gestational diabetes: the consequences of not treating. Am. J. Obstet. Gynecol. 2005;192:989–997. doi: 10.1016/j.ajog.2004.11.039. [DOI] [PubMed] [Google Scholar]
- Legardeur H., Girard G., Journy N., Ressencourt V., Durand-Zaleski I., Mandelbrot L. Factors predictive of macrosomia in pregnancies with a positive oral glucose challenge test: importance of fasting plasma glucose. Diabete Metab. 2014;40:43–48. doi: 10.1016/j.diabet.2013.01.008. [DOI] [PubMed] [Google Scholar]
- Logan K.M., Gale C., Hyde M.J., Santhakumaran S., Modi N. Diabetes in pregnancy and infant adiposity: systematic review and meta-analysis. Arch. Dis. Child Fetal Neonatal Ed. 2016 doi: 10.1136/archdischild-2015-309750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metzger B.E., Lowe L.P., Dyer A.R., Trimble E.R., Chaovarindr U., Coustan D.R., Hadden D.R., Mccance D.R., Hod M., Mcintyre H.D., Oats J.J., Persson B., Rogers M.S., Sacks D.A. Hyperglycemia and adverse pregnancy outcomes. N. Engl. J. Med. 2008;358:1991–2002. doi: 10.1056/NEJMoa0707943. [DOI] [PubMed] [Google Scholar]
- Mortensen H.B., Molsted-Pedersen L., Kuhl C., Backer P. A screening procedure for diabetes in pregnancy. Diabete Metab. 1985;11:249–253. [PubMed] [Google Scholar]
- Obstetrics Subgroup, Chinese Society of Obstetrics and Gynecology, Chinese Medical Association, Group of Pregnancy with Diabetes Mellitus, Chinese Society of Perinatal Medicine, Chinese Medical Association Diagnosis and therapy guideline of pregnancy with diabetes mellitus. Zhonghua Fu Chan Ke Za Zhi. 2014;49:561–569. [PubMed] [Google Scholar]
- Perucchini D., Fischer U., Spinas G.A., Huch R., Huch A., Lehmann R. Using fasting plasma glucose concentrations to screen for gestational diabetes mellitus: prospective population based study. BMJ. 1999;319:812–815. doi: 10.1136/bmj.319.7213.812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poomalar G.K., Rangaswamy V. A comparison of fasting plasma glucose and glucose challenge test for screening of gestational diabetes mellitus. J. Obstet. Gynaecol. 2013;33:447–450. doi: 10.3109/01443615.2013.771156. [DOI] [PubMed] [Google Scholar]
- Radaelli T., Lepercq J., Varastehpour A., Basu S., Catalano P.M., Hauguel-De Mouzon S. Differential regulation of genes for fetoplacental lipid pathways in pregnancy with gestational and type 1 diabetes mellitus. Am. J. Obstet. Gynecol. 2009;201:209.e1–209.e10. doi: 10.1016/j.ajog.2009.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trujillo J., Vigo A., Duncan B.B., Falavigna M., Wendland E.M., Campos M.A., Schmidt M.I. Impact of the International Association of Diabetes and Pregnancy Study Groups criteria for gestational diabetes. Diabetes Res. Clin. Pract. 2015;108:288–295. doi: 10.1016/j.diabres.2015.02.007. [DOI] [PubMed] [Google Scholar]
- WHO . 2013. Diagnostic Criteria and Classification of Hyperglycaemia First Detected in Pregnancy. Geneva. [PubMed] [Google Scholar]
- Zamzami T.Y.Y. Gestational diabetes in a high-risk population: perinatal outcomes with impaired fasting plasma glucose. JKAU: Med. Sci. 2007;14:49–59. [Google Scholar]
- Zhang X., Decker A., Platt R.W., Kramer M.S. How big is too big? The perinatal consequences of fetal macrosomia. Am. J. Obstet. Gynecol. 2008;198(517):e1–e6. doi: 10.1016/j.ajog.2007.12.005. [DOI] [PubMed] [Google Scholar]
- Zhu W.W., Fan L., Yang H.X., Kong L.Y., Su S.P., Wang Z.L., Hu Y.L., Zhang M.H., Sun L.Z., Mi Y., Du X.P., Zhang H., Wang Y.H., Huang Y.P., Zhong L.R., Wu H.R., Li N., Wang Y.F., Kapur A. Fasting plasma glucose at 24–28 weeks to screen for gestational diabetes mellitus: new evidence from China. Diabetes Care. 2013;36:2038–2040. doi: 10.2337/dc12-2465. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material