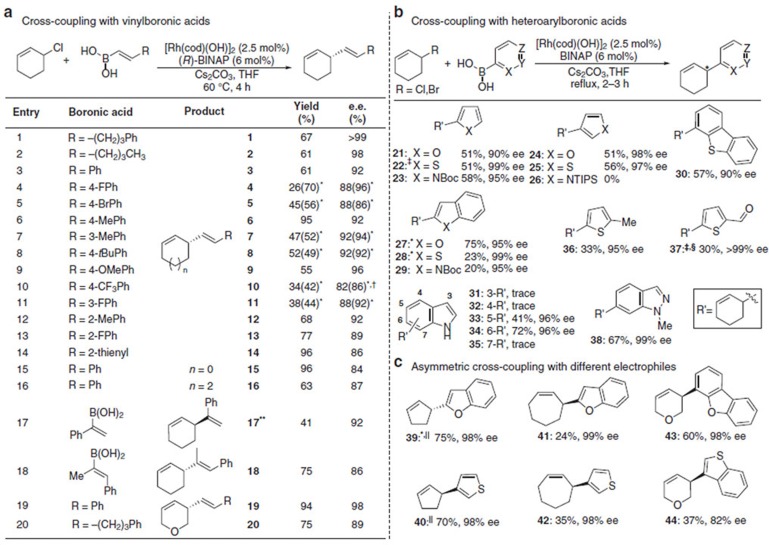
Figure 2. Asymmetric Suzuki-Miyaura coupling using vinyl- and heteroaryl boronic acids.
(a) Cross-coupling vinylboronic acids to cyclic allyl chlorides. Conditions: 0.4 mmol of allyl chloride, 0.8 mmol of vinylboronic acid, [Rh(cod)(OH)]2 (2.5 mol%), ligand (6 mol%), Cs2CO3 (1.0 eq) in THF at 60 °C. (b) Testing various heteroaromatic boronic acids in asymmetric cross-coupling. (c) Hetereoaryl boronic acids used in combination with different allyl chlorides. Conditions: 0.4 mmol of allyl chloride, 1.2 mmol of heteroaryl boronic acid, [Rh(cod)(OH)]2 (2.5 mol%), ligand (6 mol%), Cs2CO3 (1.00 eq) in THF at reflux. *In these experiments Xyl-P-PHOS was used instead of BINAP. †It is difficult to determine the ee of product 10 and the ee values here have an estimated error of ±10%. ‡Allyl bromide was used instead of allyl chloride. §The reaction was performed at r.t. for 16 h. ‖In these experiments 5 mol% [Rh(cod)(OH)]2 and 12 mol% ligand was used and the reaction stirred for four hours at reflux while protected from light. All yields are isolated yields. Enantiomeric excesses determined by HPLC, GC or SFC using a chiral non-racemic stationary phase. **Reaction using (S)-BINAP and stirred at room temperature 48 h.