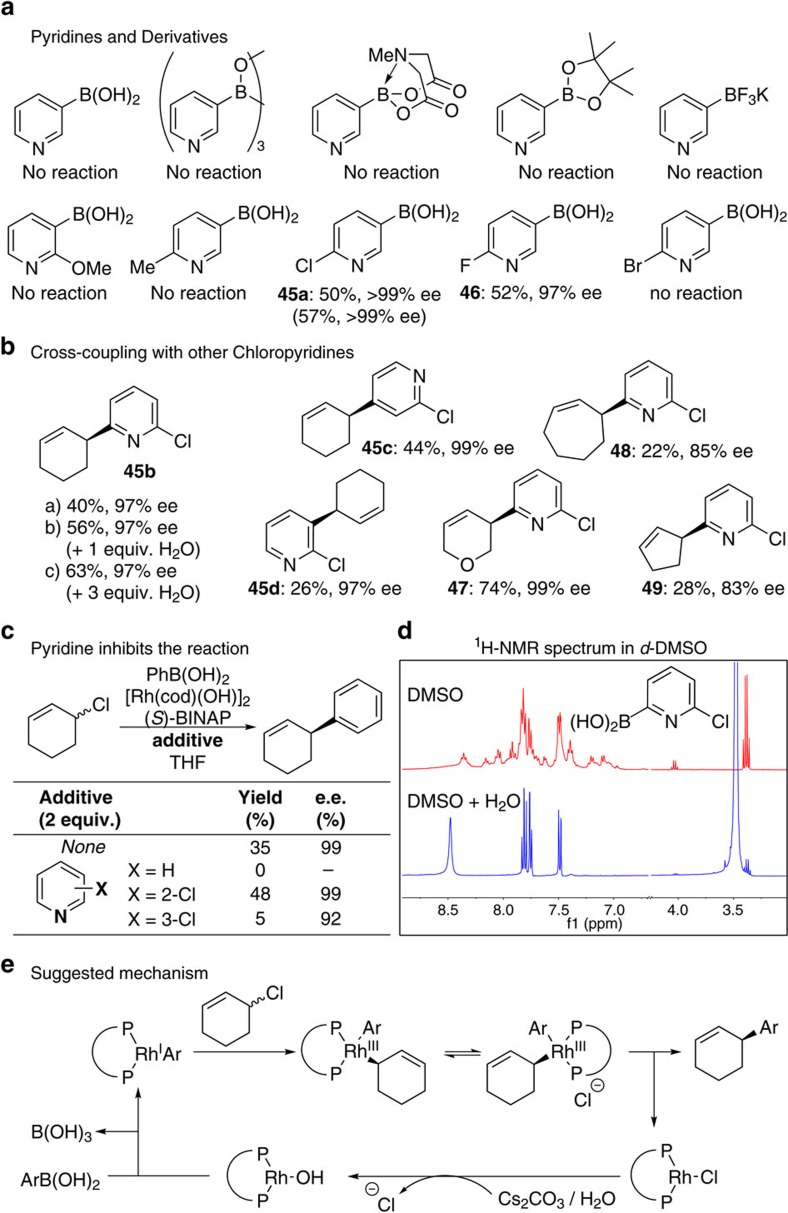
Figure 3. Asymmetric Suzuki-Miyaura coupling with pyridine-derived boronic acids.
(a) Examination of pyridine boronic acid derivatives and core-modified pyridyl boronic acids. (b) Cross-coupling of various chloropyridineboronic acids. (c) Pyridine inhibits an asymmetric coupling reaction while 2-Cl-pyridine does not, suggesting that the role of the 2-Cl-unit is to make the pyridine-based partner less Lewis basic and bind less effectively to Rh-species. (d) Addition of water to the 2-Cl-pyridinyl boronic acid simplifies its NMR spectra in DMSO suggesting it breaks aggregates into monomers. (e) Tentative reaction mechanism for the Rh-catalyzed cross-coupling. All yields are isolated yields.