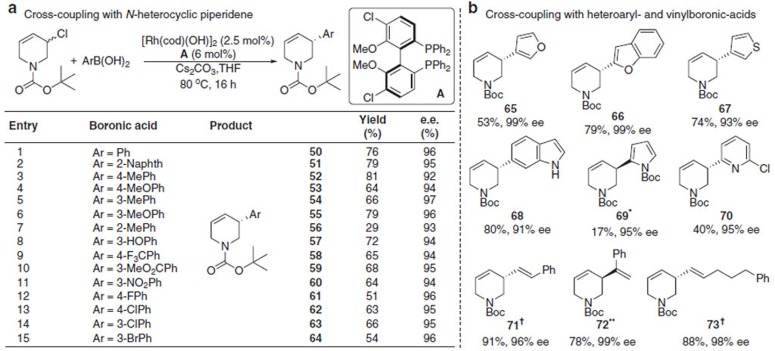
Figure 4. Coupling piperidenyl derivatives.
(a) Addition of arylboronic acids to N-Boc-3-chloro-4-piperidene. Conditions: piperidinyl chloride (1.0 equiv.), boronic acid (2.0 equiv.), [Rh(cod)(OH)]2 (2.5 mol%), A (6 mol%), Cs2CO3 (1.0 equiv.) in THF at 80 °C with stirring for 16 h. Enantiomeric excess determined by HPLC using a chiral non-racemic stationary phase. (b) Addition of heteroaromatic- and vinylboronic acids to N-Boc-3-chloro-4-piperidene. *(S)-BINAP was used instead of (R)-A. †(R)-BINAP was used instead of (R)-A. All yields are isolated yields. **Reaction using (S)-A run and stirred at room temperature 72 h.