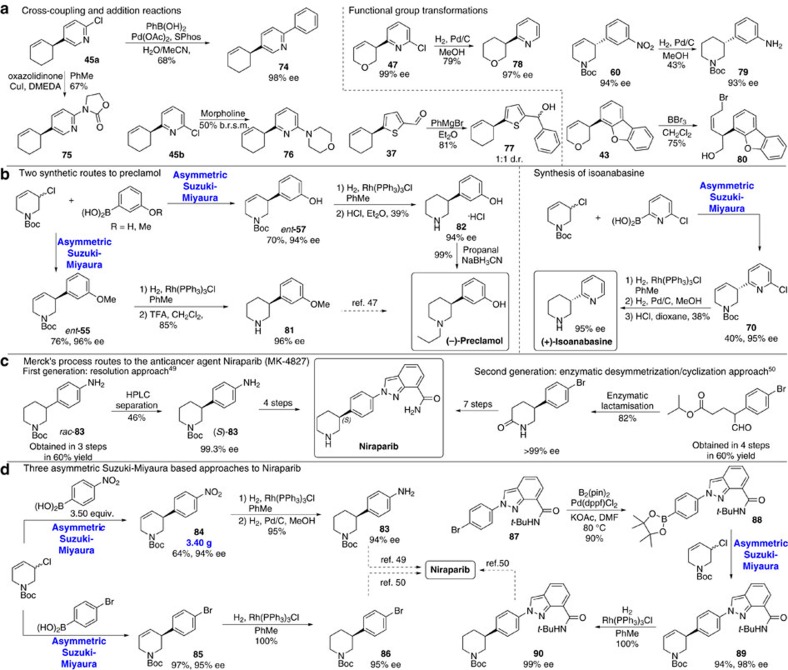
Figure 5. Further transformation of asymmetric Suzuki-Miyaura products and applications in drug and natural product synthesis.
(a) Representative transformations of different asymmetric coupling products: cross-coupling and SNAr reactions with 2-pyridine derivatives, nucleophile addition to an aldehyde, dehalogenation/hydrogenation, chemoselective ring opening with BBr3 and nitro-group reduction/hydrogentation. (b) Application of the method to the synthesis of the drug (−)-preclamol and the natural product (+)-isoanabasine. (c) Syntheses of the anticancer agent niraparib by Merck. Merck’s first process route involved resolution of racemic starting material by HPLC. Their second-generation route used an enzymatic lactamization approach to provide enantiomerically pure material. (d) To highlight the robustness and flexibility of asymmetric Suzuki-Miyaura coupling we used it as the key step in three different approaches to niraparib. All three syntheses intercept an intermediate reported by Merck and show high overall yields and levels of enantioselectivity. All yields are isolated yields.