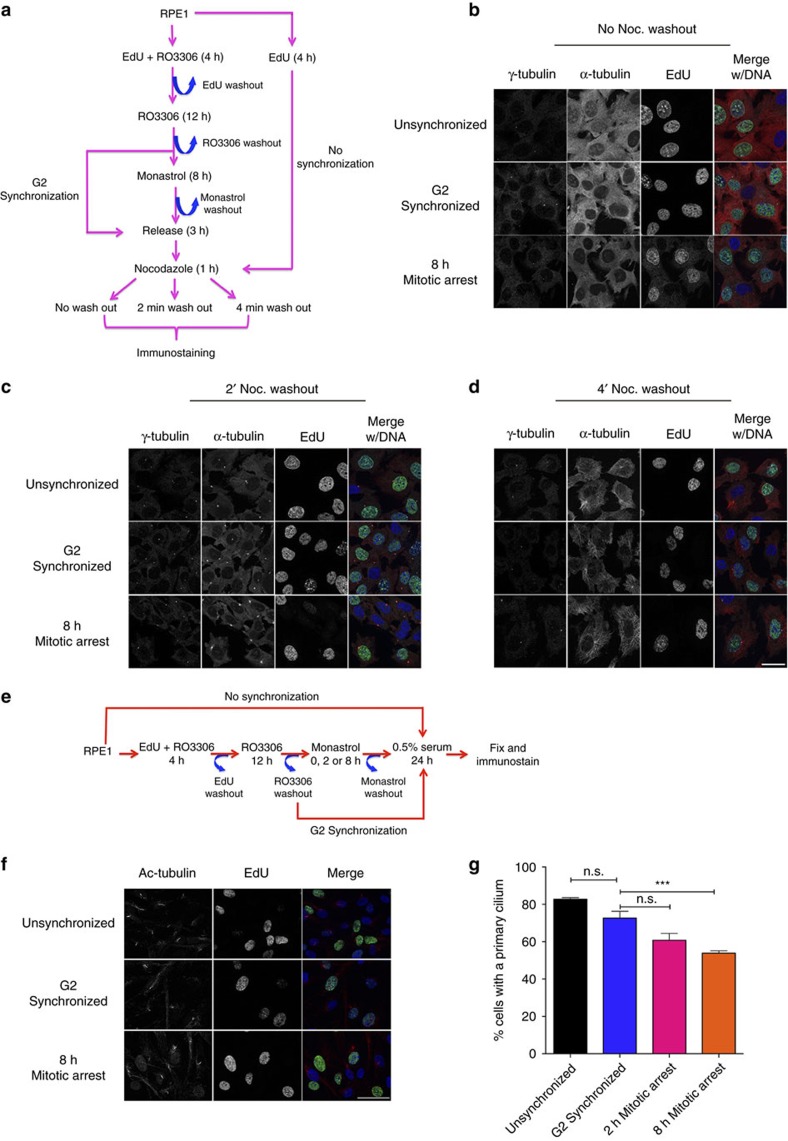
Figure 6. Centrosome function following mitotic arrest.
(a) Experimental design for microtubule regrowth assay. Unsynchronized cultures were treated with 10 μM EdU for 4 h and cultured for an additional 20 h. Alternatively, cells were treated with R03306 for 16 h to achieve G2 synchronization, and during the first 4 h of R03306 treatment, cells were pulsed with EdU. G2-synchronized or mitotically delayed cells were allowed 3 h to complete cell division. For all conditions, cultures were treated with 5 μM nocodazole for 1 h. Cells were then either fixed or washed free of nocodazole for 2–4 min before fixation to allow microtubule nucleation. Cells were then processed for EdU detection (green) and probed for γ-tubulin (cyan), α-tubulin (red) and DNA (blue). (b–d) Representative images of cells fixed either before nocodazole washout (b) or following washout for 2 min (c) or 4 min (d). Scale bar, 25 μm. (e) Experimental design. Cells were treated with 10 μM EdU for 4 h and then fixed 24 h later. Alternatively, cells were treated with R03306 for 16 h to achieve G2 synchronization, and were pulsed with EdU during the first 4 h of treatment. G2-synchronized cells were then either permitted to progress through cell division or delayed in mitosis for 8 h. For all conditions, cells were then serum starved following mitosis to induce primary cilia formation. (f) Presence of primary cilium (red) in cells that incorporated EdU (green). Scale bar, 50 μm. (g) Quantification of primary cilium in EdU-labelled cells. Error bars represent s.e.m. from three replicate experiments, 500 cells scored per condition per experiment. Significance was determined by one-way ANOVA with Tukey–Kramer post hoc test, ***P≤0.001.