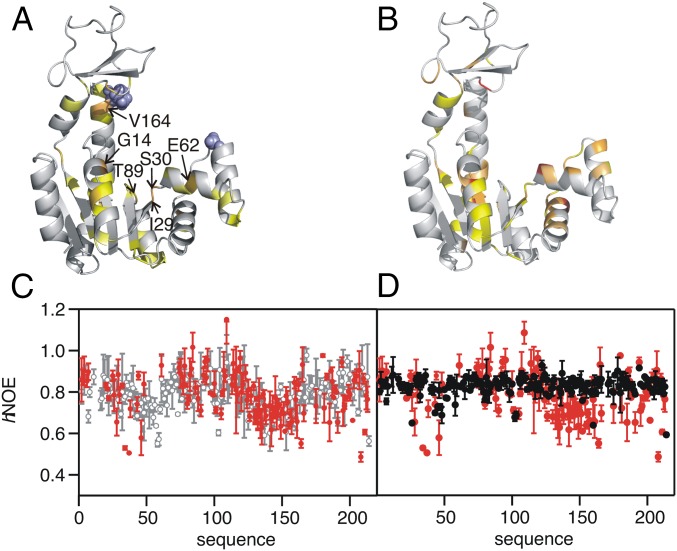
Fig. 3.
Structural and dynamic features of AdKcc under oxidative and reducing conditions. (A) Change of weighted 1H-15N chemical shifts, Δω, induced by adding TCEP to AdKcc,ox. Cysteine mutation sites are shown by blue spheres. Color coding: Δω > 0.2 ppm, light orange; 0.15 < Δω < 0.2 ppm, yellow orange; 0.1 < Δω < 0.15 ppm, yellow; 0.07 < Δω < 0.1 ppm, pale yellow. Residues with most substantial changes in chemical shifts are indicated (compare Fig. 2A) (B) Change of Δω induced by adding Ap5a to wild-type AdK. Color coding: Δω > 0.8 ppm, red; 0.6 < Δω < 0.8 ppm, bright orange; 0.4 < Δω < 0.6 ppm, light orange; 0.3 < Δω < 0.4 ppm, dark yellow. (C) hNOE for 15N nuclei of AdKcc under oxidative (red) and reducing conditions (gray). (D) hNOE for 15N nuclei of AdKcc,ox (red) and wild-type AdK in complex with Ap5a (black).