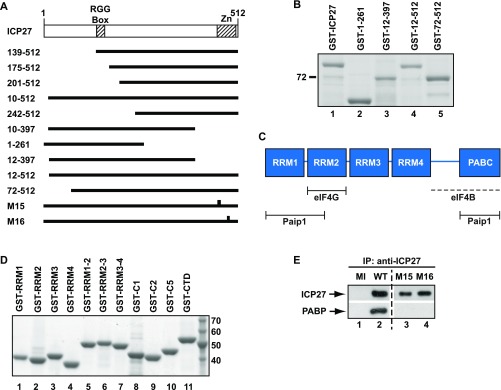
Fig. S3.
ICP27 requires PABP binding to activate translation. (A) ICP27 deletion and point mutations used in this study. (Upper) Full-length ICP27 consists of 512 amino acids. RGG-box, RNA-binding domain; Zn, C-terminal zinc finger region. (Lower) Horizontal bars and gaps indicate amino acids present and absent, respectively. Vertical bars in M15 and M16 indicate the positions of point mutations. (B) Purification of GST fusions of ICP27 truncations. Recombinant proteins were expressed from the plasmids described in Table S2 and purified from E. coli BL21 (DE3) pLysS by affinity chromatography, resolved by SDS/PAGE, and visualized with Gelcode Blue. Molecular masses (kilodaltons) are indicated. (C) PABP contains four functionally nonequivalent RRM domains and a C-terminal region composed of a proline-rich linker (line) and the PABC domain. Minimal mapped binding regions for translation initiation factors are indicated (ref. 20 and references therein). Interaction with eIF4B requires integrity of the PABP C-terminal region (dotted line). (D) GST fusions of different PABP domains purified from the plasmids listed in Table S2 as described in Fig. S1. (E) PABP coimmunoprecipitates with WT, but not M15/M16 mutant ICP27 from HSV-1–infected cell extracts. HSV-1–infected cell extracts were incubated anti-ICP27 monoclonal antibodies 1113 and 1119. ICP27 expression (Upper) and coimmunoprecipitated PABP (Lower) were detected by immunoblotting. The dashed vertical line indicates removal of irrelevant lanes from the gel.