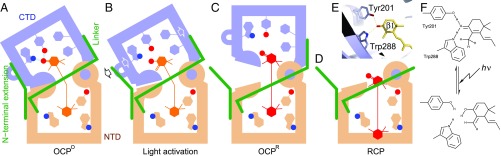
Fig. 4.
A model for OCP photoactivation. (A) The bent ketocarotenoid is embedded in the OCPO structure where two protein domains are tightly coupled. (B) Light activation disrupts the pigment–protein interactions at the β1 end of the chromophore, leading to partial domain separation as well as detachment of the NTE from the CTD. (C) Subsequent protein structural changes accompany relaxation of the carotenoid that adopts a more planar conformation in OCPR. (D) The carotenoid may undergo further translocation as in RCP. Short hydrogen bonds between Trp288/Tyr201 and the β1 ionone ring (E) may be disrupted by a transient shift in keto–enol equilibrium (F) due to light excitation of the conjugated carbonyl group in the β1 ring.