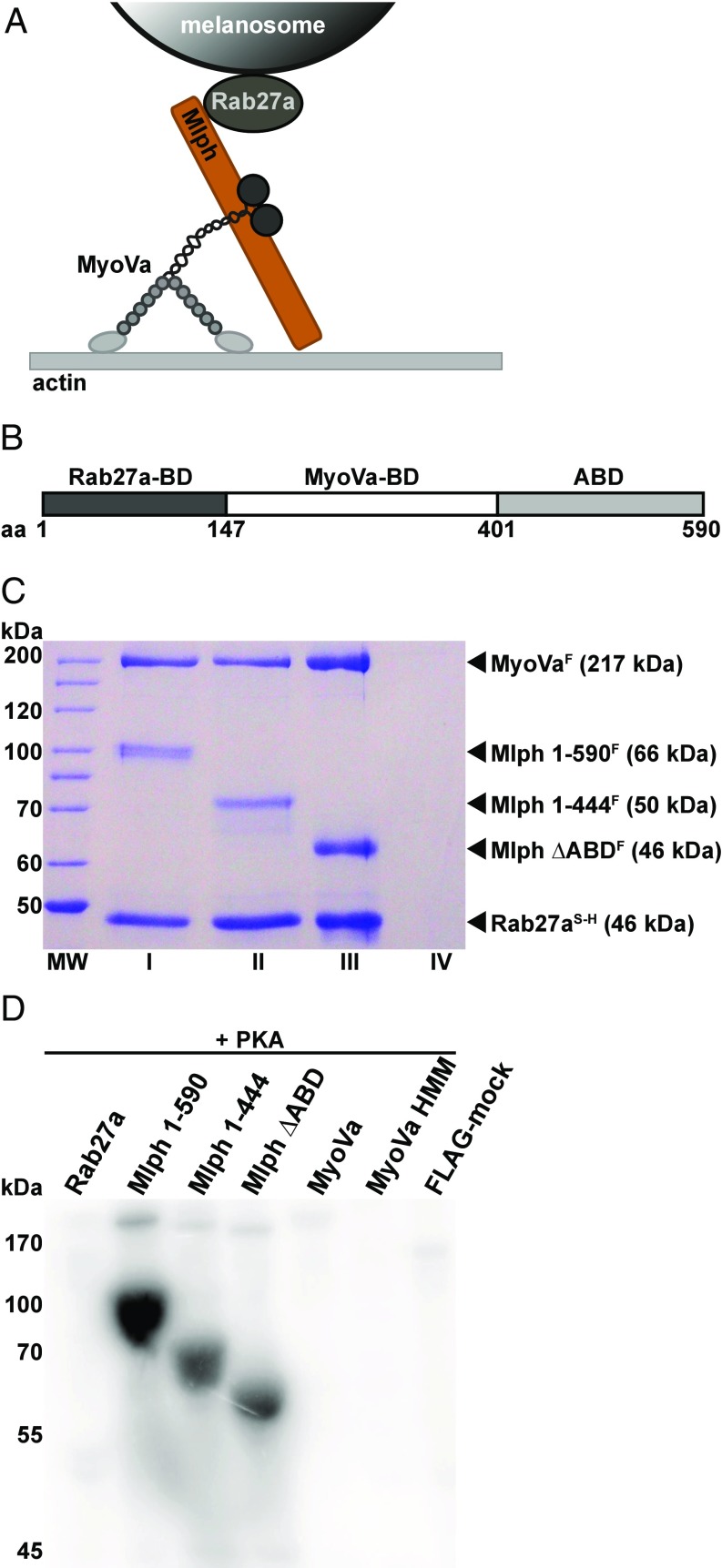
Fig. 1.
The Mlph subunit of the tripartite complex is specifically phosphorylated by PKA. (A) Schematic illustration of the MyoVa-dependent tripartite transport complex on the melanosome surface. The Rab27a GTPase resides in the melanosome membrane and recruits Mlph in a GTP-dependent manner. Mlph in turn recruits the MyoVa motor to form the tripartite transport complex. (B) Domain structure of the adaptor protein Mlph. The Rab27a-binding domain (Rab27a-BD) is located at Mlph’s N terminus (42, 43). MyoVa binds to Mlph’s middle domain with its globular tail domain and the melanocyte-specific alternatively spliced exon F (MyoVa-BD) (15, 65, 66). The C terminus of Mlph harbors an ABD (23, 24). (C) Tripartite complex reconstituted with 6×His-SNAP–tagged Rab27aS-H, FLAG-tagged MyoVaF, and full-length (lane I) or C-terminally truncated (lanes II and III) FLAG-tagged MlphF purified by Ni-NTA affinity purification. As a control for nonspecific binding, FLAG-tagged MyoVaF was also subjected to Ni-NTA affinity purification (lane IV) (Materials and Methods for details). MW, molecular mass marker. (D) The individually expressed full-length subunits of the tripartite complex along with C-terminally truncated Mlph and MyoVa HMM constructs were treated with PKA and radiolabeled ATP. Autoradiography showed specific phosphorylation of Mlph. Deletion of the C terminus of Mlph significantly decreased the phosphorylation levels. A FLAG-mock purification was included to control for unspecific phosphorylation.