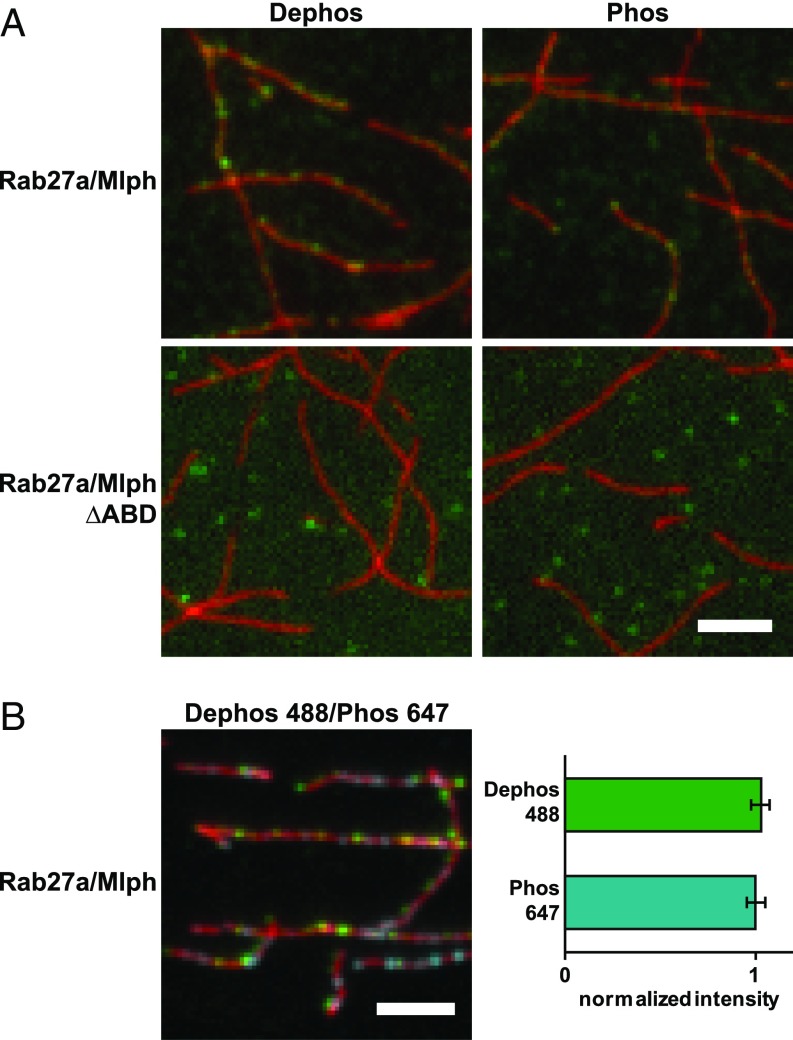
Fig. 2.
Mlph’s phosphorylation state does not interfere substantially with actin binding. (A) Actin decoration experiments were performed with surface-immobilized and Atto488-labeled actin filaments (red). Filaments were incubated with the complex formed between Mlph and Alexa Fluor 647-labeled Rab27a (green). Dephosphorylated (Dephos; Left) and the phosphorylated (Phos; Right) Mlph decorated actin filaments similarly well. Removal of the C-terminal ABD of Mlph (Rab27a/Mlph ΔABD) abolished this interaction regardless of Mlph’s phosphorylation state. (B) The dephosphorylated, Alexa Fluor 488-labeled Rab27a/Mlph complex was mixed in equal amounts with the phosphorylated, Alexa Fluor 647-labeled Rab27a/Mlph complex and was incubated with surface-attached, Atto565-labeled actin filaments. The quantification of the actin-associated fluorescence signals from the respective PKA- and phosphatase-treated Rab27a/Mlph complexes showed that the phosphorylation state of Mlph did not substantially interfere with actin binding. Error bars represent SD. (Scale bars: 3 µm.)