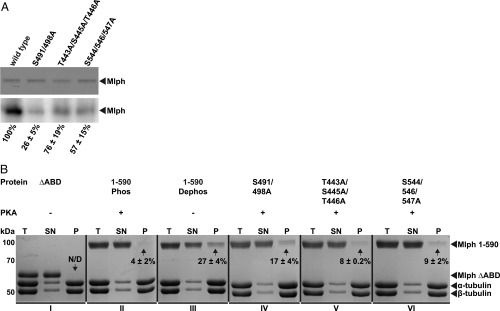
Fig. 6.
Phosphorylation of S491/498A is the main contributor to phosphorylation-dependent binding of Mlph to microtubules. (A) Nonphosphorylatable alanine mutations in three predicted phosphorylation sites reduced PKA-dependent phosphorylation. In vitro phosphorylation assays (Lower: autoradiograph; Upper: corresponding Coomassie-stained SDS/PAGE as loading control) with wild-type Mlph and its mutants showed that the S491/498A mutant suppressed the PKA-dependent phosphorylation of Mlph more efficiently (26%) than the wild-type (100%) or mutants containing weaker and less conserved PKA consensus sites (76% and 57%). (B) Microtubule cosedimentation assays were performed side by side with the truncated Mlph that lacked its ABD (I, ΔABD), phosphorylated wild-type Mlph (II, 1–590 Phos), dephosphorylated wild-type Mlph (III, 1–590 Dephos) along with phosphorylated Mlph that carried the respective nonphosphorylatable alanine mutations (IV–VI). The total reaction (T), supernatant (SN), and pellet (P) were analyzed with SDS/PAGE. As expected from the results shown in Figs. 4 and 5, Mlph that lacked its ABD and the phosphorylated wild-type Mlph failed to display a pronounced interaction with the microtubules (N/D and 4%, I and II, respectively). Also, in line with results from Fig. 5, the dephosphorylated wild-type Mlph and all rescue mutants displayed significant pelleting with microtubules [27%, 17%, 8%, and 9% (III–VI), respectively]. The mutant S491/498A containing the strong and most conserved PKA consensus site S498 also demonstrated the most pronounced effect of rescue (IV). Percentages are obtained from two independent assays ± SD. N/D, not determinable.