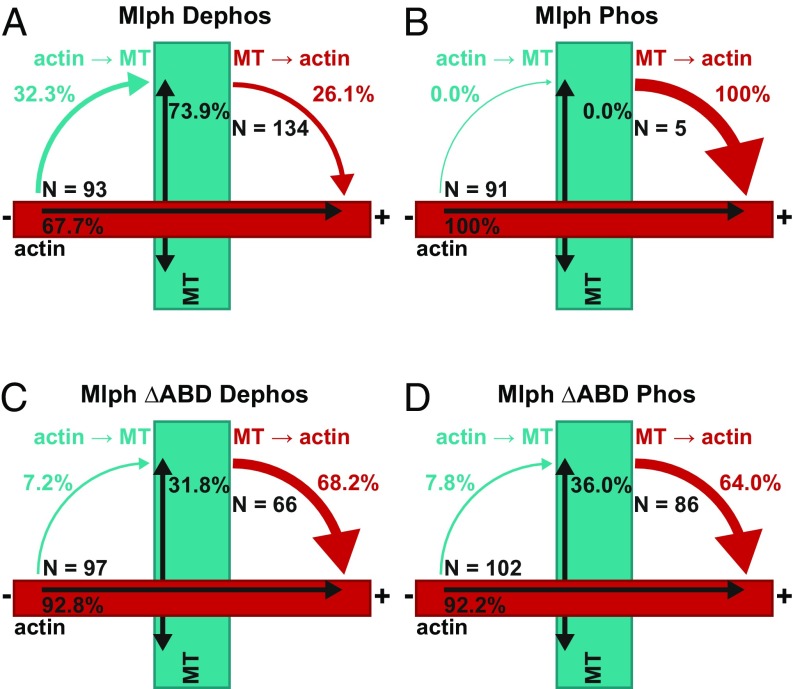
Fig. 8.
Phosphorylation state of Mlph’s ABD dictates the directionality of switching at the actin–microtubule intersections. The movement of the tripartite complex on actin and microtubules is represented by single- and double-headed black arrows, respectively. Cyan arrows depict switching from actin to microtubules; red arrows indicate switching from microtubules to actin. (A and B) The tripartite complex reconstituted with dephosphorylated Mlph displayed a significantly higher probability of switching from actin to microtubules at the interfilament intersections (A). Although 32.3% of complexes switched from actin to microtubules (67.7% continued directional movement on actin; A), the propensity of switching from actin to microtubules was abolished when the tripartite complex was assembled with phosphorylated Mlph (B). The phosphorylated complex completely ignored the interfilament intersections (0% switching) and continued its directional movement on the actin (100%; B). Conversely, dephosphorylated Mlph significantly suppressed the probability of the complex switching from microtubules to actin (26.1%; A) compared with the complex built with phosphorylated Mlph (100%; B). Indeed, the phosphorylated complex rarely interacted with the microtubules, substantially decreasing the probability of switching events from microtubules to actin (Movie S1). (C and D) In contrast, tripartite complexes assembled with dephosphorylated Mlph ΔABD (C) and phosphorylated Mlph ΔABD (D) displayed similar probabilities of switching between the two filament types, confirming that phosphorylation outside Mlph’s ABD does not interfere with the switching behavior of the tripartite complex. N indicates the number of events for each switching direction.