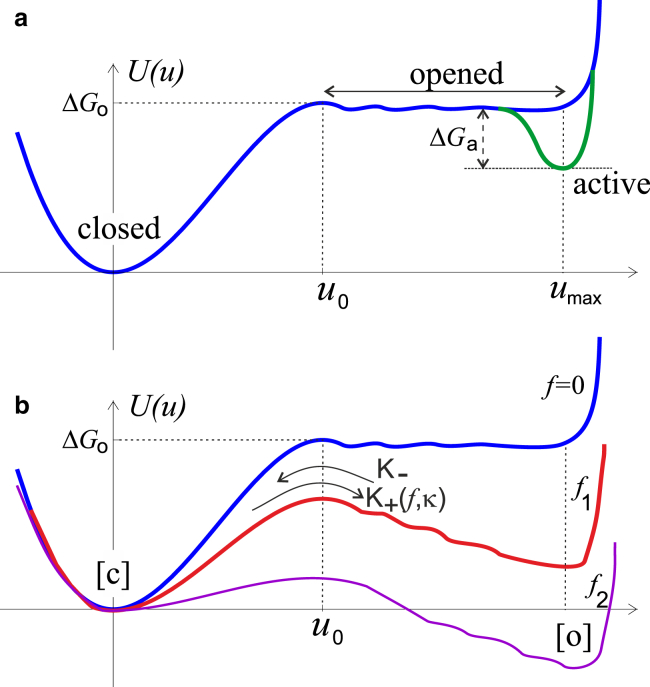
Figure 3.
Shown here is a schematic of potential energy of different FAK conformations. (a) The force-free molecule has its native folded state [c] (compare with Fig. 2). The binding free energy ΔGo has to be overcome to separate the kinase from the FERM domain, after which there is a range of conformations of where roughly the same energy is achieved by further separating these two domains in the open state [o]. At full separation (distance umax) the Src binding and kinase phosphorylation lead to the active state [a] of the protein, with the free energy gain ΔGa. (b) When a pulling force is applied to this system (f2 > f1 > 0), the potential energy profile distorts, so that both [o] and [a] states shift down in energy by the same amount of −f × umax. To see this figure in color, go online.