Abstract
Human papillomavirus (HPV) can induce cervical intraepithelial neoplasia (CIN). Vaccination against HPV can play an important role in CIN prevention. This study aims to estimate the efficacy of L1 protein vaccines (Cervarix and Gardasil) in CIN 1, 2, 3 risk reduction using meta-analysis. Relevant articles were identified by two independent researchers searching international databanks. After application of inclusion/exclusion criteria and quality assessment, eligible articles were entered into the final meta-analysis. Inverse variance method and fixed effect model were used to combine the results of the primary studies. The heterogeneity between the results was assessed using Cochrane and I2 indices. Of 11,530 evidence identified during the primary search, three papers were found eligible for meta-analysis, including 7213 participants in the intervention groups and 7170 healthy controls. The efficacy (95% confidence interval) of HPV 6, 11, 16, 18 monovalent and quadrivalent vaccines against CIN 1, CIN 2, and CIN 3 were estimated as of 95% (88–98), 97% (85–99), and 95% (78–99), respectively. This study showed that L1 protein vaccines Cervarix and Gardasil are highly protective vaccines playing an effective role in the prevention of HPV 6, 11, 16, 18 which are responsible for CIN 1, CIN 2, and CIN 3.
Keywords: Cervical intraepithelial neoplasia, efficacy, human papillomavirus, meta-analysis, vaccine
Introduction
Human papillomavirus (HPV) is one of the most important risk factors of cervical cancer which is responsible for 15,000 deaths per year in the world. This virus is also a risk factor for breast cancer[1,2] and cervical intraepithelial neoplasia (CIN).[3] CIN is often removed by the immune system, however, in some cases, it will lead to cervical cancer.[4] CIN is categorized into CIN 1, CIN 2, and CIN 3. CIN 1 is the initial phase of cellular growth abnormality and is removed by immune system. CIN 3 progresses to cervical carcinoma, and CIN 2 is the intermediate situation of them. It is generally believed that chronic cervical infection with HPV which is transmitted due to sexual contact is the main cause of CIN.[5,6]
Cervical cancer is related to the HPV types. More than 200 HPV genotypes have been identified, forty of which are transmitted through sexual contact. Among them, types 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 73 and 82 are high-risk types.[7,8] It has been shown that high-risk types of HPV 16, 18 are responsible for 70% of cervical cancers, anogenital warts, and CIN 1, 2.[9] Several studies have investigated the association between HPV (6, 11, 16, and 18) and CIN 1, 2, 3.[2,10,11,12,13] In 50%–60% of CIN cases, some evidence of HPV 16, 18 have been reported.[14] Moreover, 25% of CIN cases are associated with HPV 16 or HPV 6, 11.[15,16]
HPV is controlled by Cervarix and Gardasil vaccines which are available from 2007.[17] Cervarix containing lipid A and aluminum hydroxide is a vaccine against HPV 16 and HPV 18. Gardasil is combined with aluminum hydroxyl phosphate adjuvant, covering HPV 6, 11, 16, and 18. These vaccines induce the immune system against HPV 6, 11, 16, and 18 and some another types.[18,19,20] It has been reported that vaccination program among women under 18 reduces the incidence severe forms of CIN 2 leading to decrease in morbidity and mortality in the world.[21]
Considering the increasing prevalence of cervical cancer and the important role of vaccination in prevention of cervical cancer, this study aims to estimate the efficacy of L1 protein vaccines (Cervarix and Gardasil) against HPV 6, 11, 16, and 18 in the prevention of CIN 1, 2, and 3 using meta-analysis.
Procedure
Search strategy
To find evidence published until February 3, 2016, international databanks such as Cochrane, PubMed, Web of Science, Scopus, and Google Scholar were searched. Relevant keywords, including Cervarix, Gardasil, protein L1, CIN, clinical trial, efficacy, effectiveness, odds ratio, risk ratio, and HPV in combination with operators such as “OR” and “AND” were used for search strategy. We also reviewed all references to increase the search sensitivity. The search was conducted by two independent researchers during February 10–22, 2016, and any disagreement was managed by a third researcher.
Study selection
Full texts or abstracts of all evidence identified during the comprehensive search were extracted. First, duplicates were removed. Then, titles, abstracts, and full texts were investigated, respectively, to identify and exclude irrelevant articles. We also reviewed the results of the studies in detail to omit - if any - repeated evidence.
Inclusion criteria
Clinical trials investigating the efficacy of L1 protein vaccines Cervarix and Gardasil against CIN 1, 2, and 3 among healthy HPV-negative women with a history of three dose vaccination were selected for meta-analysis.
Exclusion criteria
Studies did not report sample size, abstracts presented in congresses without full text, nonrandomized controlled trials, and studies did not achieve enough quality scores were excluded from the meta-analysis.
Quality assessment
Two authors (A.B and M.M) evaluated the quality of the included trials using the Jadad score. This checklist is a five-point scale for assessing the quality of randomized trials. A score of three points or more indicates high quality.[22,23] Quality assessment was performed by two independent reviewers. In the case of any disagreement, further investigation and final decision-making would be carried out by a third researcher.
Data extraction
Required information extracted from studies were author's name, date and country of publication, sample size of intervention and control groups, HPV types, type of protein used, vaccination dosage, number of sexual partners, age of vaccination, number of events in each group, and follow-up time.
Statistical analysis
Data analysis was performed by Stata version 11 package (StataCorp, College Station, TX, USA). A contingency table was designed for each primary study including intervention/control groups’ data. Point estimates were combined according to the inverse variance method. The heterogeneity between the results was checked using Cochrane (Q) test and I2 index. Because of nonsignificant heterogeneity, fixed effect model was applied for combining the primary estimates. The pooled estimate of the risk ratios for reduction of cervical cancer risk with 95% confidence interval (CI) was illustrated by forest plots. This plot contained multiple boxes crossed by lines indicating study weight and 95% CIs, respectively. In addition, Egger test was used to investigate the publication bias. P < 0.1 was considered statistically significant.
Results
Of 11,530 articles identified during the primary search, 1702 duplicates were removed and 9453 papers were excluded after limiting the search strategy. Reviewing titles and abstracts, 240 papers were identified irrelevant, and investigating the full texts of the rest papers, 132 irrelevant studies were detected. Finally, three papers were considered eligible for meta-analysis [Figure 1].
Figure 1.
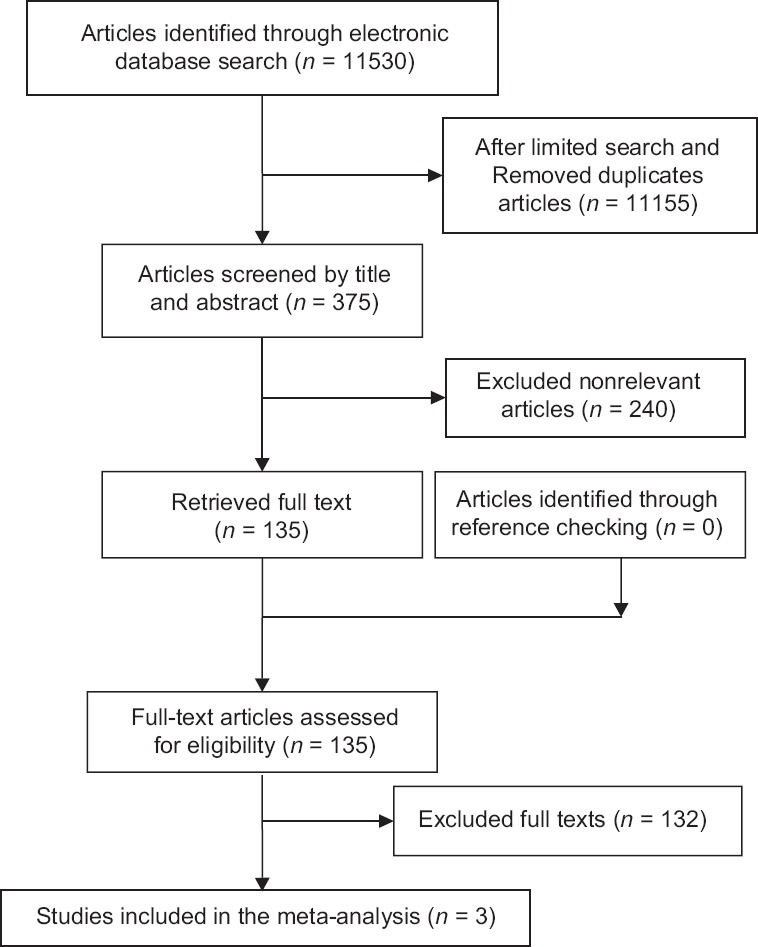
Flowchart of study selection
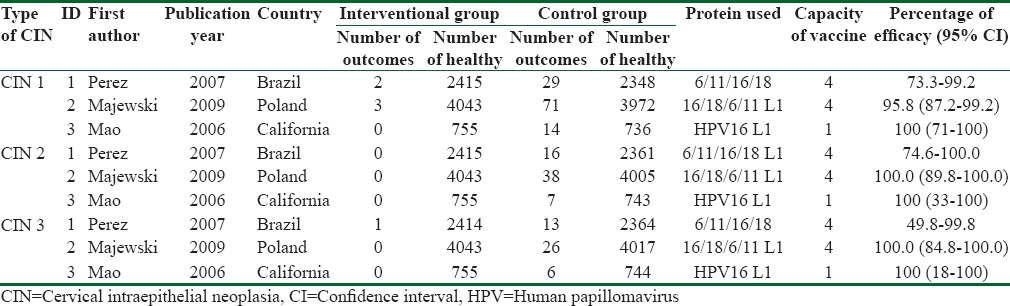
These three studies were carried out in Brazil (2007), Poland (2009), and California, USA (2006). All of them investigated the CIN 1, CIN 2, and CIN 3 as the outcome of interest. Totally, 14,383 participants were investigated aged between 16 and 26 years. The average number of sexual partners in these studies was <5. Follow-up times in Brazil, Poland, and California studies were 7, 36, and 48 months, respectively. All participants had received three vaccine doses. Type of vaccines applied in the first two studies was quadrivalent (HPV 6, 11, 16, 18 L1) and in the study carried out in the USA was monovalent (HPV 16 L1). Table 1 shows the efficacy of vaccines according to the type of study and vaccine.
Table 1.
Characteristics of primary studies according to cervical intraepithelial neoplasia type, vaccine type, and efficacy
The heterogeneity indices showed low heterogeneity between the primary results regarding the efficacy of both vaccines against CIN 1 (I2 = 0; Q = 0.32, P = 0.8). Therefore, fixed effect model was applied for combining the results. The pooled risk ratio for developing CIN 1 in the intervention and control groups was estimated as of 0.05 (95% CI: 00.02–0.12) [Figure 2]. It means that the efficacy of Cervarix and Gardasil for reducing the risk of CIN 1 is 95% (95% CI: 88%–98%). Egger test showed no evidence of publication bias (β = −0.2; P = 0.9).
Figure 2.
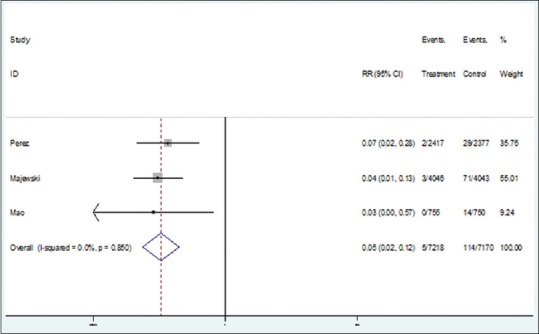
Forest plot for the efficacy of vaccines against cervical intraepithelial neoplasia 1 based on risk ratio
Heterogeneity indices for primary results for CIN 2 were not statistically significant (I2 = 0, Q = 0.64; P = 0.7). Therefore, using fixed effect model, the pooled RR for CIN 2 risk reduction by monovalent and quadrivalent vaccines was estimated as of 0.03 (95% CI: 0.01–0.15) [Figure 3]. In other words, the efficacy of vaccine in reducing the risk of CIN 2 was 97% (95% CI: 85%–99%). In addition, Egger test showed nonsignificant publication bias (β =42.9, P = 0.13).
Figure 3.
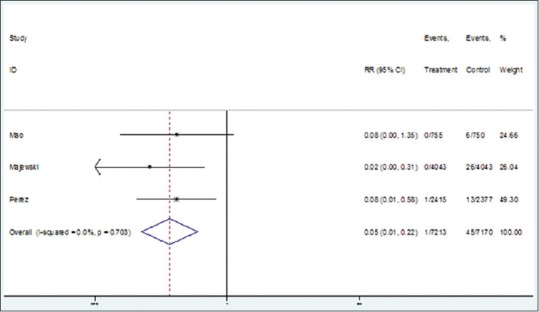
Forest plot for the efficacy of vaccines against cervical intraepithelial neoplasia 3 based on risk ratio
There was no significant heterogeneity between the results of primary studies regarding the effect of monovalent and quadrivalent vaccines against HPV 6, 11, 16, 18, and CIN 3 (I2 = 0, Q = 0.70; P = 0.7). The pooled risk ratio for CIN 3 using Cervarix and Gardasil was estimated as of 0.05 (95% CI: 0.01–0.22) [Figure 3] indicating a 95% (95% CI: 87%–99%) efficacy. Moreover, the Egger test results showed no evidence of publication bias (β = 1.6; P = 0.6).
Conclusions
Our meta-analysis showed that the efficacy of HPV L1 protein vaccines (Cervarix and Gardasil) in reducing the risk of CIN 1, CIN 2, and CIN 3 was more than 95%.
As reported in the literature, HPV 16 and HPV 18 are responsible for 86% of adenocarcinomas. Although HPV 16/HPV 18-associated CIN is not common, vaccination plays a very important role in the prevention of this malignancy. Therefore, vaccination program can reduce the prevalence of the adenocarcinoma.[24,25] The highest risk of HPV infection is occurred in the first 5–10 years after sexual contact.[26,27,28] In most countries, the first sex experience begins during the age of 15–17 years. In the study carried out by Block et al., the efficacy of vaccination was estimated as of 98%–99%. The concentration of the antibody after vaccination was higher in girls aged 10–15 years than those aged 16–23 years. Thus, the vaccination program before the first sexual contact is more effective in the prevention of cervical cancer.[29]
Gardasil quadrivalent vaccine has been approved by European countries and is being used in many populations in different ages. For example, in France, Australia, Luxembourg, and Sweden, people are vaccinated in ages 14, 16–26, 13–16, and 10–11, respectively. It indicates that vaccination is an important factor in the prevention of genital disorders among HPV noninfected women and girls. Majewski showed that the quadrivalent vaccine caused approximately 100% decrease in the incidence of cervical cancer and genital diseases which are related to HPV (6, 11, 16, and 18). Investigations conducted in European countries showed that vaccination plays an important prophylactic role for CIN 1 and CIN 3 in women with four or less sexual partners. They also found that vaccination prevented the CIN 2 more than 56%.[30]
The HPV 16 L1 vaccine is not only involved in the prevention of HPV 16 infection but also plays an important role in CIN 2, 3 prevention. The protection period for this vaccine against HPV 16 is reported as of 3.5 years. According to the results of the previous studies, HPV11 vaccine can induce a 100% protection against CIN 2, 3 during 3.5 years among noninfected women receiving at least one dose vaccine. It can lead to a lower incidence of cervical cancer within the community.[31,32]
The results of a survey conducted among Danish women with normal cytology showed the incidence of HPV 6, 11, 16, 18 as of 7.8%.[33] If all HPV types were considered, the incidence could be 14%. According to the results of the other studies, HPV types other than HPV 16 and HPV 18 are responsible for 20% of cervical cancers. Also, vaccination is not covered all HPV types, and cytological screening of populations is recommended.[34]
Unfortunately, we used only three primary studies in our meta-analysis. However, since these studies are clinical trials with limited ethical and methodological issues, performing meta-analysis using low numbers of primary studies would be important. The strength of this study is the high agreement and low heterogeneity between the results. Therefore, the effect of the limited sample size on the total estimates is low. In case of adding more primary studies to the meta-analysis, no considerable changes would be observed in the pooled estimates. Our primary studies did not report the time periods of vaccines efficacy. Due to this limitation, we could not estimate the immunization period.
Our study revealed that using quadrivalent and monovalent L1 protein Cervarix and Gardasil have high protective effects against HPV 6, 11, 16, and 18 infections which are main risk factors for CIN 1, 2, and 3. Therefore, implementation of regular vaccination programs against HPV (6, 11, 16, and 18) can play a critical role in the prevention of CIN 1, 2, and 3. The current meta-analysis provides appropriate evidence for policymaking about vaccination program planning.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Haghshenas MR, Mousavi T, Moosazadeh M, Afshari M. Human papillomavirus and breast cancer in Iran: A meta- analysis. Iran J Basic Med Sci. 2016;19:231–7. [PMC free article] [PubMed] [Google Scholar]
- 2.Malary M, Moosazadeh M, Hamzehgardeshi Z, Afshari M, Moghaddasifar I, Afsharimoghaddam A. The prevalence of cervical human papillomavirus infection and the most at-risk genotypes among Iranian healthy women: A systematic review and meta-analysis. Int J Prev Med. 2016;7:70. doi: 10.4103/2008-7802.181756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cho HJ, Oh YK, Kim YB. Advances in human papilloma virus vaccines: A patent review. Expert Opin Ther Pat. 2011;21:295–309. doi: 10.1517/13543776.2011.551114. [DOI] [PubMed] [Google Scholar]
- 4.Ault KA, Giuliano AR, Edwards RP, Tamms G, Kim LL, Smith JF, et al. A phase I study to evaluate a human papillomavirus (HPV) type 18 L1 VLP vaccine. Vaccine. 2004;22:3004–7. doi: 10.1016/j.vaccine.2004.02.020. [DOI] [PubMed] [Google Scholar]
- 5.Park J, Sun D, Genest DR, Trivijitsilp P, Suh I, Crum CP. Coexistence of low and high grade squamous intraepithelial lesions of the cervix: Morphologic progression or multiple papillomaviruses? Gynecol Oncol. 1998;70:386–91. doi: 10.1006/gyno.1998.5100. [DOI] [PubMed] [Google Scholar]
- 6.Agorastos T, Miliaras D, Lambropoulos AF, Chrisafi S, Kotsis A, Manthos A, et al. Detection and typing of human papillomavirus DNA in uterine cervices with coexistent grade I and grade III intraepithelial neoplasia: Biologic progression or independent lesions? Eur J Obstet Gynecol Reprod Biol. 2005;121:99–103. doi: 10.1016/j.ejogrb.2004.11.024. [DOI] [PubMed] [Google Scholar]
- 7.de Sanjose S, Quint WG, Alemany L, Geraets DT, Klaustermeier JE, Lloveras B, et al. Human papillomavirus genotype attribution in invasive cervical cancer: A retrospective cross-sectional worldwide study. Lancet Oncol. 2010;11:1048–56. doi: 10.1016/S1470-2045(10)70230-8. [DOI] [PubMed] [Google Scholar]
- 8.Clifford GM, Gallus S, Herrero R, Muñoz N, Snijders PJ, Vaccarella S, et al. Worldwide distribution of human papillomavirus types in cytologically normal women in the International Agency for Research on Cancer HPV prevalence surveys: A pooled analysis. Lancet. 2005;366:991–8. doi: 10.1016/S0140-6736(05)67069-9. [DOI] [PubMed] [Google Scholar]
- 9.Harper DM. Currently approved prophylactic HPV vaccines. Expert Rev Vaccines. 2009;8:1663–79. doi: 10.1586/erv.09.123. [DOI] [PubMed] [Google Scholar]
- 10.Smith JS, Lindsay L, Hoots B, Keys J, Franceschi S, Winer R, et al. Human papillomavirus type distribution in invasive cervical cancer and high-grade cervical lesions: A meta-analysis update. Int J Cancer. 2007;121:621–32. doi: 10.1002/ijc.22527. [DOI] [PubMed] [Google Scholar]
- 11.Insinga RP, Dasbach EJ, Elbasha EH, Liaw KL, Barr E. Progression and regression of incident cervical HPV 6, 11, 16 and 18 infections in young women. Infect Agent Cancer. 2007;2:15. doi: 10.1186/1750-9378-2-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Clifford GM, Rana RK, Franceschi S, Smith JS, Gough G, Pimenta JM. Human papillomavirus genotype distribution in low-grade cervical lesions: Comparison by geographic region and with cervical cancer. Cancer Epidemiol Biomarkers Prev. 2005;14:1157–64. doi: 10.1158/1055-9965.EPI-04-0812. [DOI] [PubMed] [Google Scholar]
- 13.Haghshenas M, Golini-Moghaddam T, Rafiei A, Emadeian O, Shykhpour A, Ashrafi GH. Prevalence and type distribution of high-risk human papillomavirus in patients with cervical cancer: A population-based study. Infect Agent Cancer. 2013;8:20. doi: 10.1186/1750-9378-8-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Balbi G, Napolitano A, Giordano F, Capuano S, Manganaro MA, Di Martino L, et al. Role of the association of high-risk HPV identified by real-time PCR in cervical preneoplastic lesions. Eur J Gynaecol Oncol. 2012;33:467–71. [PubMed] [Google Scholar]
- 15.Greer CE, Wheeler CM, Ladner MB, Beutner K, Coyne MY, Liang H, et al. Human papillomavirus (HPV) type distribution and serological response to HPV type 6 virus-like particles in patients with genital warts. J Clin Microbiol. 1995;33:2058–63. doi: 10.1128/jcm.33.8.2058-2063.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li HX, Zhu WY, Xia MY. Detection with the polymerase chain reaction of human papillomavirus DNA in condylomata acuminata treated with CO2 laser and microwave. Int J Dermatol. 1995;34:209–11. doi: 10.1111/j.1365-4362.1995.tb01572.x. [DOI] [PubMed] [Google Scholar]
- 17.Stanley MA. Human papillomavirus vaccines. Rev Med Virol. 2006;16:139–49. doi: 10.1002/rmv.498. [DOI] [PubMed] [Google Scholar]
- 18.Brown DR, Kjaer SK, Sigurdsson K, Iversen OE, Hernandez-Avila M, Wheeler CM, et al. The impact of quadrivalent human papillomavirus (HPV; types 6, 11, 16, and 18) L1 virus-like particle vaccine on infection and disease due to oncogenic nonvaccine HPV types in generally HPV-naive women aged 16-26 years. J Infect Dis. 2009;199:926–35. doi: 10.1086/597307. [DOI] [PubMed] [Google Scholar]
- 19.Harper DM, Franco EL, Wheeler CM, Moscicki AB, Romanowski B, Roteli-Martins CM, et al. Sustained efficacy up to 4 5 years of a bivalent L1 virus-like particle vaccine against human papillomavirus types 16 and 18: Follow-up from a randomised control trial. Lancet. 2006;367:1247–55. doi: 10.1016/S0140-6736(06)68439-0. [DOI] [PubMed] [Google Scholar]
- 20.Stanley M, Gissmann L, Nardelli-Haefliger D. Immunobiology of human papillomavirus infection and vaccination – Implications for second generation vaccines. Vaccine. 2008;26(Suppl 10):K62–7. doi: 10.1016/j.vaccine.2008.05.066. [DOI] [PubMed] [Google Scholar]
- 21.Pollock KG, Kavanagh K, Potts A, Love J, Cuschieri K, Cubie H, et al. Reduction of low- and high-grade cervical abnormalities associated with high uptake of the HPV bivalent vaccine in Scotland. Br J Cancer. 2014;111:1824–30. doi: 10.1038/bjc.2014.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, et al. Assessing the quality of reports of randomized clinical trials: Is blinding necessary? Control Clin Trials. 1996;17:1–12. doi: 10.1016/0197-2456(95)00134-4. [DOI] [PubMed] [Google Scholar]
- 23.Li J, Liu Z, Chen R, Hu D, Li W, Li X, et al. The quality of reports of randomized clinical trials on traditional Chinese medicine treatments: A systematic review of articles indexed in the China National Knowledge Infrastructure database from 2005 to 2012. BMC Complement Altern Med. 2014;14:362. doi: 10.1186/1472-6882-14-362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Castellsagué X, Díaz M, de Sanjosé S, Muñoz N, Herrero R, Franceschi S, et al. Worldwide human papillomavirus etiology of cervical adenocarcinoma and its cofactors: Implications for screening and prevention. J Natl Cancer Inst. 2006;98:303–15. doi: 10.1093/jnci/djj067. [DOI] [PubMed] [Google Scholar]
- 25.Garland SM, Hernandez-Avila M, Wheeler CM, Perez G, Harper DM, Leodolter S, et al. Quadrivalent vaccine against human papillomavirus to prevent anogenital diseases. N Engl J Med. 2007;356:1928–43. doi: 10.1056/NEJMoa061760. [DOI] [PubMed] [Google Scholar]
- 26.Moscicki AB. Genital HPV infections in children and adolescents. Obstet Gynecol Clin North Am. 1996;23:675–97. [PubMed] [Google Scholar]
- 27.Collins S, Mazloomzadeh S, Winter H, Blomfield P, Bailey A, Young LS, et al. High incidence of cervical human papillomavirus infection in women during their first sexual relationship. BJOG. 2002;109:96–8. doi: 10.1111/j.1471-0528.2002.01053.x. [DOI] [PubMed] [Google Scholar]
- 28.Winer RL, Lee SK, Hughes JP, Adam DE, Kiviat NB, Koutsky LA. Genital human papillomavirus infection: Incidence and risk factors in a cohort of female university students. Am J Epidemiol. 2003;157:218–26. doi: 10.1093/aje/kwf180. [DOI] [PubMed] [Google Scholar]
- 29.Block SL, Nolan T, Sattler C, Barr E, Giacoletti KE, Marchant CD, et al. Comparison of the immunogenicity and reactogenicity of a prophylactic quadrivalent human papillomavirus (types 6, 11, 16, and 18) L1 virus-like particle vaccine in male and female adolescents and young adult women. Pediatrics. 2006;118:2135–45. doi: 10.1542/peds.2006-0461. [DOI] [PubMed] [Google Scholar]
- 30.Majewski S, Bosch FX, Dillner J, Iversen OE, Kjaer SK, Muñoz N, et al. The impact of a quadrivalent human papillomavirus (types 6, 11, 16, 18) virus-like particle vaccine in European women aged 16 to 24. J Eur Acad Dermatol Venereol. 2009;23:1147–55. doi: 10.1111/j.1468-3083.2009.03266.x. [DOI] [PubMed] [Google Scholar]
- 31.Mao C, Koutsky LA, Ault KA, Wheeler CM, Brown DR, Wiley DJ, et al. Efficacy of human papillomavirus-16 vaccine to prevent cervical intraepithelial neoplasia: A randomized controlled trial. Obstet Gynecol. 2006;107:18–27. doi: 10.1097/01.AOG.0000192397.41191.fb. [DOI] [PubMed] [Google Scholar]
- 32.Schiffman MH. Recent progress in defining the epidemiology of human papillomavirus infection and cervical neoplasia. J Natl Cancer Inst. 1992;84:394–8. doi: 10.1093/jnci/84.6.394. [DOI] [PubMed] [Google Scholar]
- 33.Kjaer SK, Breugelmans G, Munk C, Junge J, Watson M, Iftner T. Population-based prevalence, type- and age-specific distribution of HPV in women before introduction of an HPV-vaccination program in Denmark. Int J Cancer. 2008;123:1864–70. doi: 10.1002/ijc.23712. [DOI] [PubMed] [Google Scholar]
- 34.Kjaer SK, van den Brule AJ, Paull G, Svare EI, Sherman ME, Thomsen BL, et al. Type specific persistence of high risk human papillomavirus (HPV) as indicator of high grade cervical squamous intraepithelial lesions in young women: Population based prospective follow up study. BMJ. 2002;325:572. doi: 10.1136/bmj.325.7364.572. [DOI] [PMC free article] [PubMed] [Google Scholar]


