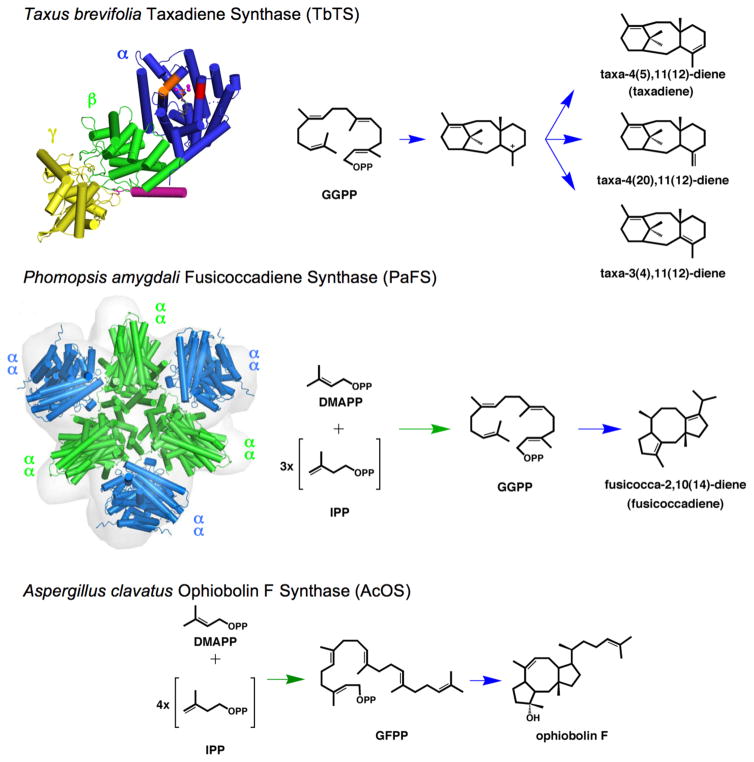
Figure 1. Domain architecture in αβγ and αα diterpene synthases.
Taxadiene synthase from Taxus brevifolia (TbTS) catalyzes the cyclization of GGPP to form taxa-4(5),11(12)-diene as the major product (henceforth designated simply “taxadiene”). Alternative proton elimination steps yield taxa-4(20),11(12)-diene and taxa-3(4),11(12)-diene as minor products. This cyclization occurs exclusively in the α domain (blue); neither the β domain (green) nor the γ domain (yellow) contain a functional active site. A polypeptide segment connected to the N-terminal helix of the β-domain (magenta) is believed to help cap the active site in the α domain during catalysis. Fusicoccadiene synthase from Phomopsis amygdali (PaFS) is a hexamer of subunits with αα domain architecture. The chain elongation reaction of DMAPP and 3 IPP molecules is catalyzed in the C-terminal GGPP synthase α domain (green), and the GGPP cyclization reaction forming fusicoccadiene is catalyzed in the N-terminal cyclase α domain (blue). Although neither the tertiary structure nor the quaternary structure of ophiobolin F synthase from Aspergillus clavatus (AcOS) are known, this bifunctional sesterterpene synthase also adopts an αα domain architecture in which the chain elongation reaction of DMAPP and 4 IPP molecules is catalyzed in the C-terminal α domain to form GFPP, which then undergoes cyclization in the N-terminal α domain to form ophiobolin F. Detailed catalytic mechanisms for each enzyme are found in Figure S1.