Abstract
Spinal muscular atrophy (SMA), the leading genetic disease of children, is caused by low levels of survival motor neuron (SMN) protein. Here, we employ A15/283, an antisense oligonucleotide targeting a deep intronic sequence/structure, to examine the impact of restoration of SMN in a mild SMA mouse model. We show gender-specific amelioration of tail necrosis upon subcutaneous administrations of A15/283 into SMA mice at postnatal days 1 and 3. We also demonstrate that a modest increase in SMN due to early administrations of A15/283 dramatically improves testicular development and spermatogenesis. Our results reveal near total correction of expression of several genes in adult testis upon temporary increase in SMN during early postnatal development. This is the first demonstration of in vivo efficacy of an antisense oligonucleotide targeting a deep intronic sequence/structure. This is also the first report of gender-specific amelioration of SMA pathology upon a modest peripheral increase of SMN.
Keywords: spinal muscular atrophy, SMA, SMN, SMN2, ISS-N1, ISS-N2, antisense oligonucleotide
Singh and colleagues show that early postnatal peripheral administrations of an antisense oligonucleotide (ASO) that disrupts an intra-intronic RNA structure formed by a long-distance interaction confers therapeutic benefits in a mild model of spinal muscular atrophy (SMA). The authors demonstrate remarkable amelioration of the male reproductive organ phenotype upon ASO treatment. The findings underscore the importance of examining gender when assessing the therapeutic efficacy of compounds for the treatment of SMA.
Introduction
Spinal muscular atrophy (SMA), a leading genetic disease of children and infants, results from the deletion, mutation, or gene conversion of survival motor neuron 1 (SMN1).1 SMN1 serves as the major source of SMN, an essential protein that participates in small nuclear ribonucleoprotein (snRNP) biogenesis, transcription, macromolecular transport, translation, cell signaling, and stress granule formation.2 SMN2, a nearly identical paralog of SMN1 present in humans, cannot compensate for the loss of SMN1 due to SMN2 exon 7 skipping, leading to the production of SMNΔ7, a truncated and only partially stable protein.3, 4, 5, 6 The spectrum of SMA disease is broad, ranging from death in early infancy to a near-normal lifespan, in which the ability to walk is eventually lost.7 The severity of SMA correlates with SMN levels; the lower they are, the greater the severity of the disease. Patients lacking SMN1 but carrying higher SMN2 copy numbers or SMN2 mutations that lead to greater SMN production exhibit a milder phenotype.8, 9, 10, 11, 12, 13, 14, 15
Various mouse models that recapitulate different SMA severities display complex pathology manifested by the loss of lower motor neurons, defects in neuromuscular junctions, and abnormalities in peripheral tissues, including the heart, muscles, intestines, liver, and spleen.16, 17, 18, 19, 20, 21, 22, 23, 24, 25 Suggesting a gender-specific role of SMN, heterozygous mice producing low levels of SMN show incidences of male infertility.26 There are also reports of developmental defects in male reproductive organs in patients with mild SMA.27, 28 Supporting the critical role of SMN in male reproductive organ development, the testis expresses the highest level of SMN among all tissues examined.29 Consistently, we have reported severe impairment of testis development and spermatogenesis in allele C (C/C) mice, a mild SMA mouse model.29 We have also shown that the high SMN levels in the adult C/C testis is maintained at least in part due to a splicing switch that leads to the predominant inclusion of SMN2 exon 7.29 However, this switch occurs only after postnatal day 14 (P14) and cannot reverse defects produced by low SMN levels at the early stages of male reproductive organ development.29 These recent findings underscore the need for the incorporation of the reproductive organ phenotype as a gender-specific outcome measure of potential SMA therapies.
All SMA patients carry at least one copy of SMN2; hence, correction of SMN2 exon 7 splicing holds the promise for SMA therapy. An antisense oligonucleotide (ASO)-mediated correction of SMN2 exon 7 splicing offers the desirable advantage of being gene specific. We have previously reported intronic splicing silencer N1 (ISS-N1), spanning from the tenth to the 24th positions of SMN2 intron 7 as a promising therapeutic target for splicing correction.30 Thus far, ISS-N1 remains the most studied target for an ASO-mediated splicing correction in SMA (reviewed by Sivanesan et al.31 and Singh et al.32). In particular, independent studies employing early peripheral administrations of the ISS-N1-targeting ASOs have shown unprecedented therapeutic benefits on the lifespan of severe mouse models of SMA.33, 34, 35 The U.S. Food and Drug Administration (FDA) has recently approved Spinraza (nusinersen), an ISS-N1-targeting ASO, as the first medical therapy for SMA.36 This study is inspired by another antisense target, ISS-N2, located deep within SMN2 intron 7.37 Compared to an ISS-N1-targeting ASO that produces a robust increase in exon 7 inclusion, an ISS-N2-targeting ASO provides a somewhat lesser response.37 Nonetheless, ISS-N2 offers a unique opportunity to test how a moderate increase in SMN protein through targeting of a deep intronic structure would affect the phenotype of a mouse model of SMA.
Here, we examine the effect of an early peripheral treatment with an ISS-N2-targeting ASO, termed A15/283, on the phenotype of the C/C model of SMA.23 Of note, despite its relatively mild SMA phenotype, the C/C model displays a severe male reproductive organ phenotype.29 We have recently established that a high level of SMN is required during early stages of testicular development for normal development of the male reproductive organ.29 Hence, C/C mice emerged as an ideal choice to evaluate gender-specific outcome measures in mild SMA. We performed a blinded and randomized study employing subcutaneous (SC) administration of A15/283 at P1 and P3 and monitored the phenotype into adulthood. Mice receiving A15/283 showed gender-specific amelioration of tail necrosis and substantial improvement in male reproductive organ pathology. This study validates that a modest peripheral increase in SMN level at the early stage of postnatal development has a significant impact on the male reproductive organ phenotype. Our findings represent the first in vivo demonstration of therapeutic efficacy of splicing correction by targeting a deep intronic sequence in a genetic disease.
Results
Effect of Early Peripheral Administration of an ISS-N2-Targeting ASO on SMN Levels in Different Tissues of C/C Mice
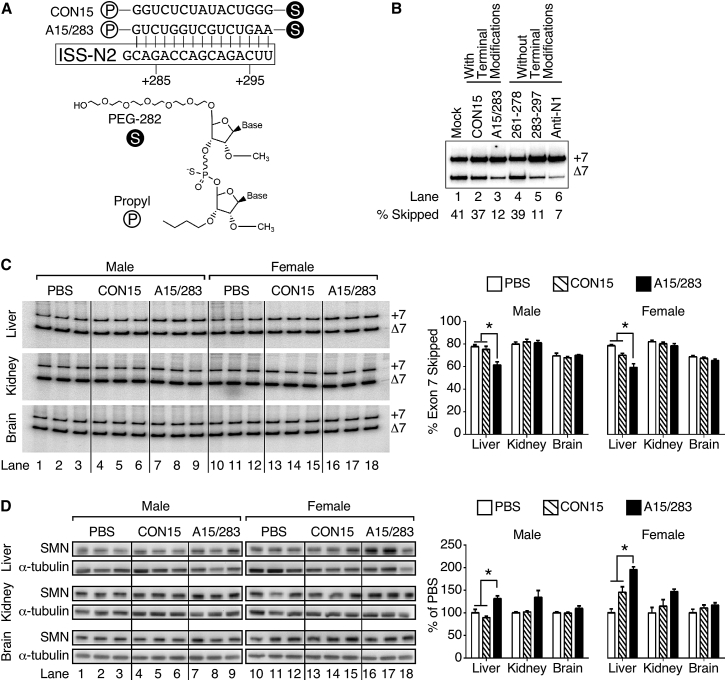
To monitor the effect of abrogation of a deep intronic structure on SMN levels in various tissues of C/C mice, we employed A15/283, a 15-nucleotide (nt)-long ISS-N2-targeting ASO (Figure 1A). As a control, we used CON15, a scrambled version of A15/283 predicted to have no complementarity in the murine genome (Figure 1A). All ASOs used in this study carried a phosphorothioate (PS) backbone, 2′-O-methyl (2OMe) modification of sugar moieties, a PEG-282 extension at the 5′-terminus, and a C3 (propyl) spacer at the 3′-terminus (Figure 1A). Similar terminal modifications have been demonstrated to improve in vivo efficacy of an ASO.38 Employing SMA patient fibroblasts, we confirmed that the incorporated terminal modifications did not affect the splice-switching ability of A15/283 (Figure 1B). On the basis of the promising results of recent studies,33, 38 we chose SC administration of A15/283 for the peripheral restoration of SMN due to SMN2 exon 7 splicing correction. Using fluorescence in situ hybridization,39 we first confirmed that the SC administration of A15/283 distributes this ASO to several peripheral tissues, including the liver, heart, and quadriceps (Figure S1). We then administered ASOs (80 μg/g body weight [BW]) subcutaneously into C/C pups at P1 and P3 and collected tissue at P7. Compared to CON15 treatment of both male and female pups, SC delivery of A15/283 modestly increased SMN2 exon 7 inclusion in the liver (Figure 1C). However, we did not observe a noticeable change in the splicing of SMN2 exon 7 in the kidney and brain. We next examined the expression of SMN2-derived SMN protein using a human SMN-specific antibody. Consistent with the increase in SMN2 exon 7 inclusion, we observed an increase in SMN protein in the liver of both A15/283-treated males and females (Figure 1D). There was no discernible change in SMN protein in the brain, but there was a detectable non-significant increase in SMN in A15/283-treated male and female kidney (Figure 1D). Overall, the above results confirmed partial restoration of SMN in a limited number of tissues upon SC administration of A15/283.
Figure 1.
Examination of the In Vivo Efficacy of A15/283, an ISS-N2-Targeting ASO
(A) The top image shows the sequence of A15/283 and its binding location to SMN2 intron 7. The sequence of the scrambled control ASO, CON15, is shown. Numbering starts from the beginning of intron 7. The circle with “S” represents a PEG-282 at the 5′ terminus and the circle with “P” represents a propyl spacer at the 3′ terminus. The bottom image presents a general schematic of A15/283 and CON15 chemistry, including a PS backbone with 2OMe modification of sugar moieties, the 5′ PEG-282 and 3′ propyl spacer. (B) Semiquantitative radioactive RT-PCR gel showing SMN2 exon 7 inclusion in SMA patient fibroblasts in the presence of different ASOs. Mock refers to cells not transfected with ASO. ASO 261-278 and 283-297 binds to nucleotides 261–278 and 283–297 in SMN2 intron 7, respectively. Anti-N1 binds to the ISS-N1 target (nucleotides 10–24) in SMN2 intron 7.30 CON15 and A15/283 had the terminal modifications described in (A). ASO 261-278, 283-297, and Anti-N1 had a PS backbone with 2OMe sugar modification but lacked the terminal modifications. The percentage of exon 7 skipping was calculated based on the total value of SMN2 exon-7-included and exon-7-skipped product. (C) Images of radioactive RT-PCR acrylamide gels showing SMN2 exon 7 inclusion in male and female P7 C/C liver, brain, and kidney after SC injection of PBS, CON15, or A15/283 on P1 and P3 (n = 3 for each tissue, treatment, and sex). Gender and treatments are indicated at the top of the images. The graphs present quantification of the gels with the percent of transcripts with exon 7 skipped shown. (D) Western blots showing full-length SMN (derived from SMN2) and α-tubulin proteins in male and female P7 C/C brain, liver, and kidney after SC injection at P1 and P3 (n = 3 for each treatment, tissue, and sex). Gender and treatments are indicated at the top of the images. The graphs present densitometry for each tissue. Error bars on the graphs indicate SEM. For (C) and (D), data for each sex and tissue were compared with a one-way ANOVA, followed by Tukey’s multiple comparison test. *p < 0.05 indicates a significant difference between the means.
Gender-Specific Amelioration of Tail Necrosis upon Early Peripheral Treatment with A15/283
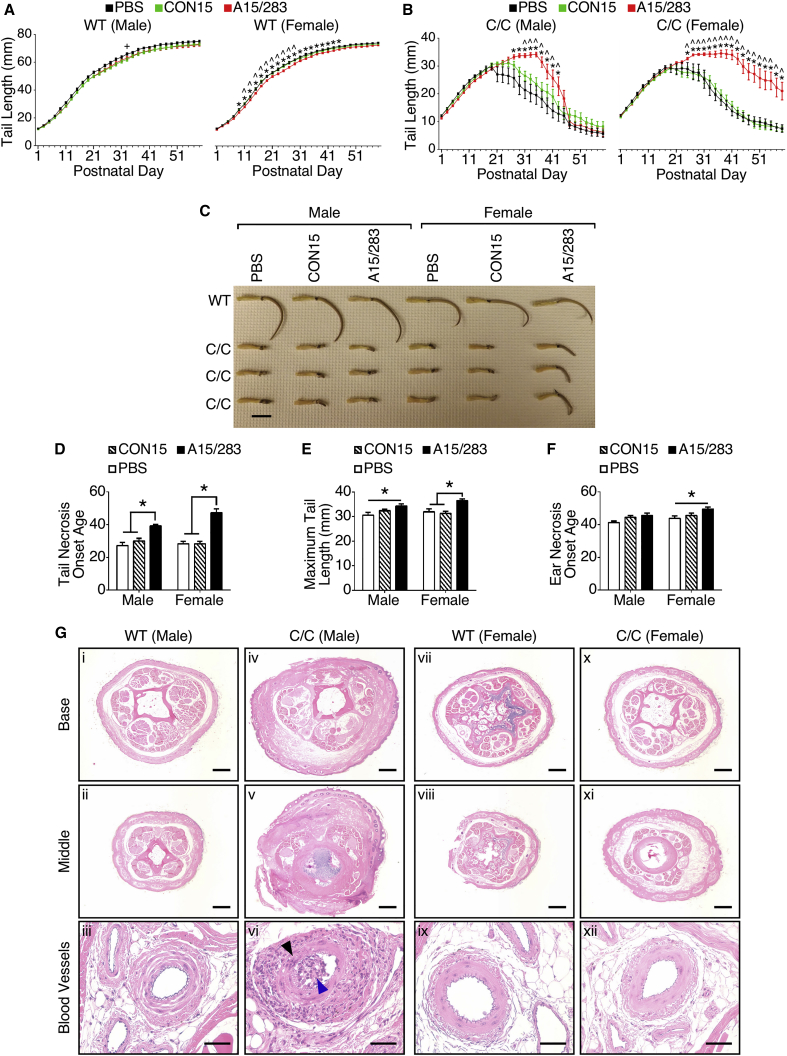
Tail necrosis is a common phenotype in mild mouse models of SMA40, 41 and is the most prominent physical phenotype of the C/C mouse.23 Tail necrosis begins within a few weeks of birth, and the tail never reaches the same length of that of wild-type (WT) mice.23, 40, 41 Because P1 and P3 SC administrations of A15/283 modestly increased SMN protein in peripheral organs (Figure 1D), we asked whether this early change in SMN level has any impact on the age of onset of tail and ear necrosis. As expected, the tail lengths of WT mice were only minimally affected by treatment with A15/283 or CON15 (Figure 2A). However, A15/283-treated C/C males had significantly longer tails than CON15-treated C/C males from P31 until P41 when the tails began to necrose (Figure 2B). The ameliorating effect of A15/283 was even more pronounced in C/C females; A15/283-treated C/C female tails were significantly longer than the ones in CON15-treated C/C females from P27 until the end of the study (Figure 2B). By P60, A15/283-treated C/C females generally maintained a short tail that appeared relatively normal except for necrosis at the tip, whereas all other C/C mice maintained only a necrotic tail stub (Figure 2C). Our results showed that A15/283 treatment significantly delayed the onset of visible tail necrosis for both male and female C/C mice and ear necrosis in females (Figures 2D and 2F). Noticeably, A15/283 treatment delayed tail necrosis onset substantially in the case of female mice compared to male mice (Figure 2D). A15/283 treatment, however, produced only a minimal effect on restoring tail length (Figure 2E). We also determined the SMN protein levels at P60 in several organs and found no appreciable difference between A15/283-treated and untreated C/C mice (Figure S2). Taken together, our results support that a modest elevation of SMN protein during early postnatal development has a gender-specific amelioration of tail necrosis in C/C mice.
Figure 2.
Early Peripheral A15/283 Treatment Provided Gender-Specific Amelioration of Tail Necrosis in C/C Mice
(A) Tail length over time for WT males injected with PBS (n = 9), CON15 (n = 17), and A15/283 (n = 23) (left) and WT females injected with PBS (n = 15), CON15 (n = 9), and A15/283 (n = 20) (right). (B) Tail length over time for C/C males injected with PBS (n = 9), CON15 (n = 11), and A15/283 (n = 7) (left) and C/C females injected with PBS (n = 11), CON15 (n = 14), and A15/283 (n = 12) (right). (C) Photograph of male and female WT and C/C tails at P60. Treatments are indicated above each column of tails. Scale bar, 25 mm. (D) Tail necrosis onset age for C/C mice. (E) Maximum tail length for C/C mice. (F) Ear necrosis onset age for C/C mice. (G) H&E-stained tail cross sections from male and female P60 mice injected with A15/283 at P1 and P3. The sex and genotype are indicated above each micrograph. Base refers to a cross section taken from the base of the tail near the body and middle refers to a cross section taken at the middle of the remaining tail stub. Micrographs with blood vessels show a higher magnification of the median caudal vasculature at the base of the tail. Black arrowhead indicates blood vessel muscle disorganization, and blue arrowhead indicates blood vessel obstruction. Base and middle micrographs scale bar, 200 μm; blood vessels micrographs scale bar, 50 μm. See also Figure S3 for tail micrographs from PBS-injected and CON15-treated mice. Error bars on graphs indicate SEM. For (A) and (B), data were analyzed with a two-way repeated-measures ANOVA followed by Tukey’s multiple comparison test. + indicates a significant difference between PBS and CON15, * indicates a significant difference between PBS and A15/283, and ˆ indicates a significant difference between CON15 and A15/283. For (D–F), data were analyzed by a two-way ANOVA followed by Tukey’s multiple comparison test. *p < 0.05 indicates a significant difference between the means.
To further explore why A15/283 treatment had a gender-specific effect on tail necrosis in C/C mice, we evaluated tail cross sections at P60. In particular, we focused on the vasculature and muscle fibers at the base and the visually necrotic middle of the remaining tail stub. Despite the delayed onset in tail necrosis (Figures 2B and 2D), the tail stubs from A15/283-treated C/C males at P60 exhibited a generally swollen appearance, with notable degeneration at both the base and the middle area of the tail (Figure 2G, panels iv–vi). The vasculature at the base of the necrotic tail had disorganized muscle fiber bundles (Figure 2G, black arrowhead in panel vi) and obstructed vessel lumen (Figure 2G, blue arrowhead in panel vi). Notably, cross sections of tail stubs from the A15/283-treated C/C female exhibited near-normal tissue architecture and showed little evidence of vascular necrosis (Figure 2G, panels x–xii). On the contrary, tails of the PBS-injected or CON15-treated C/C males and females showed evidence of extensive tissue necrosis and vascular damage (Figure S3). These results support that an early peripheral elevation of SMN promotes a long-term, gender-specific modulation of vascular necrosis in mild SMA.
C/C mice exhibit an approximately 10%–15% reduction in BW from weaning through adulthood.23 We tracked BW of the treated mice from P1 through P60 to determine if the early peripheral increase in SMN level corrects this deficit. We did not observe a noticeable effect of ASO treatment on the BW of either male or female C/C mice (Figure S4). It is likely that a substantially higher level of SMN maintained continuously would be required to improve the C/C BW phenotype.
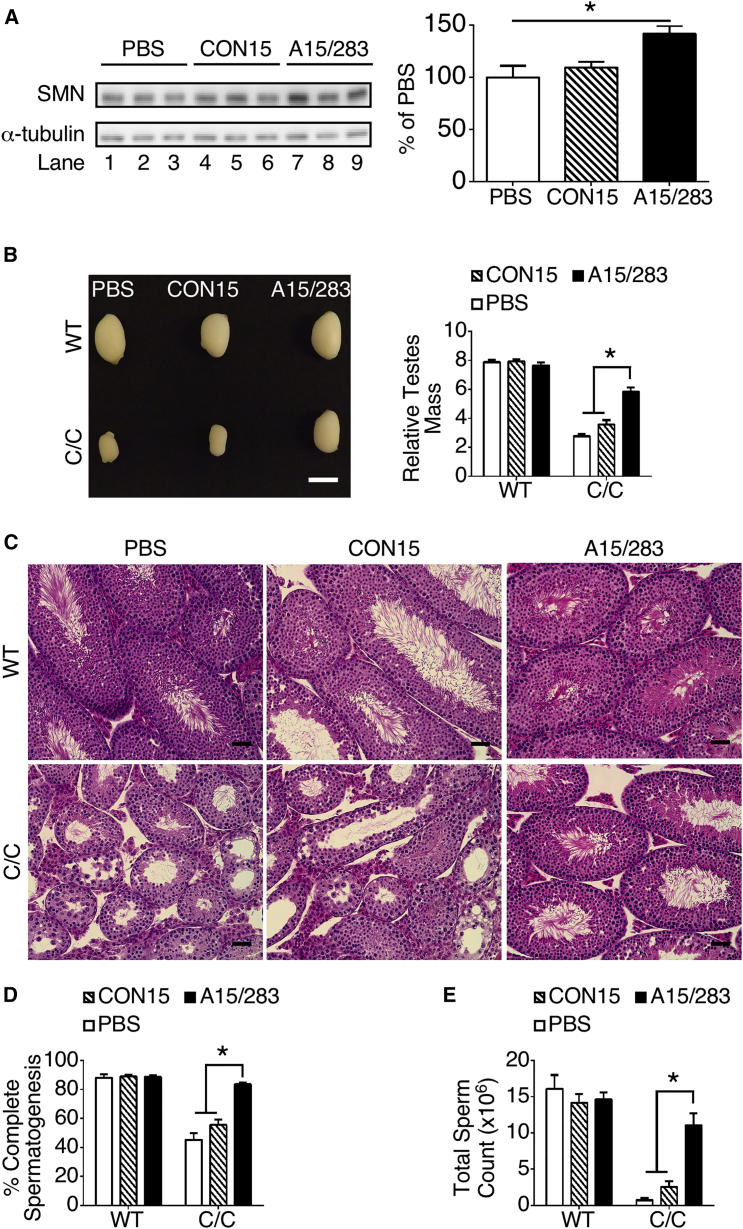
Modest Increase in SMN Protein in Testis upon Early Peripheral Treatment with A15/283 Substantially Improved Testis Development and Function
Although the SMN level in the adult C/C testis is comparable to that of the adult WT testis, the reduced SMN level in the testis of the newly born C/C pups markedly impairs testicular development.29 To assess the effect of the early peripheral increase of SMN level on testicular development of C/C males, we analyzed the adult testis upon SC administration of A15/283 at P1 and P3. We observed an approximately 40% increase in SMN protein level in testis of C/C males at P7 treated with A15/283 compared to the CON15-treated males (Figure 3A). Compared to CON15, treatment of C/C males with A15/283 at P1 and P3 increased relative testicular mass approximately 2-fold at P60 (Figure 3B). This observation underscores the fact that even a modest increase in the testis SMN level early in the postnatal period produces a long-term positive impact on mammalian testicular development. Supporting this argument, a substantially large proportion of seminiferous tubules of A15/283-treated testes versus CON15-treated C/C testes exhibited all stages of spermatogenesis (Figure 3C), and the percentage of seminiferous tubules with complete spermatogenesis was no different from that of WT testes (Figure 3D). In comparison, the majority of seminiferous tubules in CON15-treated testes lacked spermatozoa, which is indicative of incomplete spermatogenesis (Figures 3C and 3D). Consistent with the amelioration of the testis phenotype in C/C males treated with A15/283, we observed no significant difference in epididymal sperm counts between A15/283-treated C/C and WT mice (Figure 3E). We previously demonstrated that the C/C reproductive phenotype was limited to males because female C/C mice exhibited only a mild reduction in the uterus and ovary size, but were fertile.29 Regardless of treatment with A15/283 or CON15, histological analysis showed no difference between WT and C/C uterus and ovaries at P60 (Figure S5).
Figure 3.
Early Peripheral A15/283 Treatment Improved Testis Development and Normalized Spermatogenesis
(A) Western blot showing full-length SMN (derived from SMN2) and α-tubulin proteins in P7 C/C testis after SC treatment at P1 and P3 (n = 3 mice per treatment). The graph to the right presents densitometry of the blots. (B) Photograph with representative testes from WT and C/C mice treated with PBS, CON15, or A15/283. Scale bar, 500 μm. The graph to the right presents relative testes mass (mg testis per g BW) at P60. For WT mice, n = 9, 17, and 23 mice for the PBS, CON15, and A15/283 groups, respectively. For C/C mice, n = 9, 11, and 7 mice for the PBS, CON15, and A15/283 groups, respectively. (C) Representative H&E-stained P60 testis cross sections showing seminiferous tubules. Scale bar, 50 μm. (D) Percent of tubules with complete spermatogenesis from P60 mice (see Materials and Methods for further details). Numbers of mice were the same as in (B). (E) Sperm count from P60 mice. Sperm were isolated from the epididymis and counted with a hemocytometer. Numbers of mice were the same as in (B). Error bars on graphs indicate SEM. For (A), data were analyzed with a one-way ANOVA followed by Tukey’s multiple comparison test. *p < 0.05 indicates a significant difference between the means. For (B), (D), and (E), data were analyzed by a two-way ANOVA followed by Tukey’s multiple comparison test. *p < 0.05 indicates a significant difference between the means.
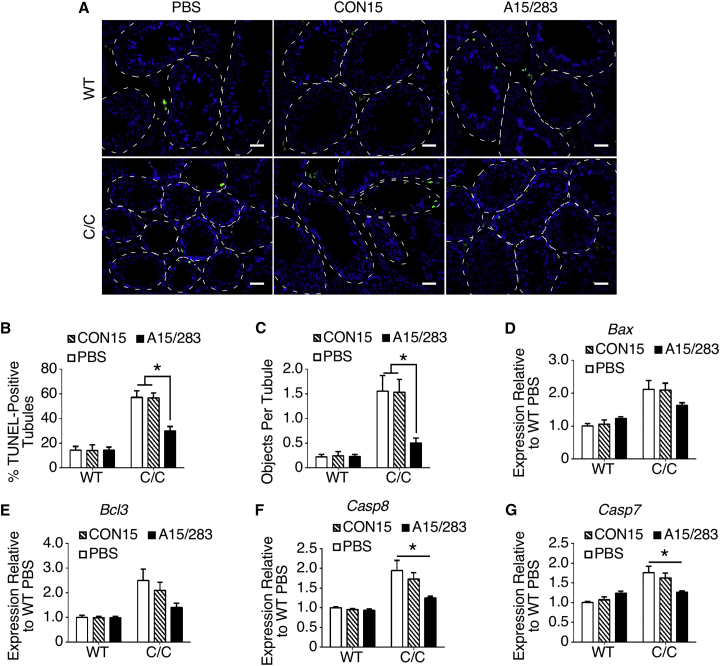
Effect of Early Peripheral Treatments with A15/283 on Apoptosis in Adult C/C Testis
Apoptotic pathways play a critical role in mammalian spermatogenesis and are adversely affected in adult C/C testis examined at P42.29 Therefore, we next tested whether the peripheral administration of A15/283 into C/C pups at P1 and P3 had a normalizing effect on apoptosis in adult C/C testis. For this, we performed terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling (TUNEL) staining using testis samples collected at P60 (Figure 4A). This technique identifies cells with DNA damage usually associated with apoptosis. Although A15/283 treatment significantly reduced the percentage of TUNEL-positive seminiferous tubules in C/C testes compared to CON15 treatment, the percentage was still significantly higher than in WT testes (Figure 4B). However, the number of apoptotic cells in TUNEL-positive tubules in A15/283-treated C/C testes was comparable to that of the WT testes (Figure 4C). These findings confirm that an increase in SMN in testis during the early developmental stage has a normalizing effect on apoptotic pathways in adult testis. As further evidence that A15/283 treatment affected apoptosis regulation, we examined levels of several pro- (Aifm1, Bax, Capn2, Casp7, Casp8, and Casp9) and anti-apoptotic (Akt and Bcl3) genes in P60 testis (Figures 4D–4G and S6). We have previously shown that all of these genes are upregulated in adult C/C testis analyzed at P42.29 A15/283 treatment restored near-normal expression of many of the apoptosis-related genes we tested, most notably Bax, Bcl3, Casp8, and Casp9 (Figures 4D–4G). Treatment also restored normal expression of Aifm1, Akt1, and Apaf1 (Figures S6A–S6C), but produced no effect on the expression of Capn2 or Casp9 (Figures S6D and SDE). Interestingly, contrary to our previous observation,29 the expression of Capn2 and Casp9 was no different in C/C testis compared to WT testis. This discrepancy could be age related because the previous study analyzed C/C testis at P42 compared to the analysis in this study at P60. Taken together with TUNEL staining, our results confirm that a modest postnatal increase in testicular SMN has a long-term benefit on regulation of apoptotic pathways in the testis of a mild mouse model of SMA.
Figure 4.
Early Peripheral A15/283 Treatment Decreased TUNEL-Positive Bodies and Normalized Expression of Apoptosis-Related Genes
(A) Representative TUNEL staining from testis cross sections. Treatment and genotype are indicated above and to the left of the micrographs, respectively. The blue signal indicates DAPI (nuclei), and the green signal indicates TUNEL-positive bodies. Seminiferous tubules are outlined with white dashed lines. Scale bar in each micrograph, 50 μm. (B) Percentage of seminiferous tubules with at least one TUNEL-positive object. For WT, n = 5, 5, and 8 mice for PBS, CON15, and A15/283 groups, respectively. For C/C mice, n = 5, 6, and 6 mice for PBS, CON15, and A15/283 groups, respectively. (C) Average number of TUNEL-positive objects per seminiferous tubule. Numbers of mice were the same as in (B). (D–G) Relative expression of pro- and anti-apoptotic genes in testis. Expression for each gene is relative to the WT PBS group (set at 1.0). For WT, n = 6, 7, and 5 mice for PBS, CON15, and A15/283 groups. For C/C, n = 5, 5, and 4 mice for PBS, CON15, and A15/283 groups, respectively. See also Figure S6 for expression of additional pro- and anti-apoptotic genes. Error bars on graphs indicate SEM. For (B–G), data were analyzed with a two-way ANOVA followed by Tukey’s multiple comparisons test. *p < 0.05 indicates a significant difference between the means.
Early Peripheral Treatments with A15/283 Substantially Reduces the Perturbations in Gene Expression in Adult C/C Testis
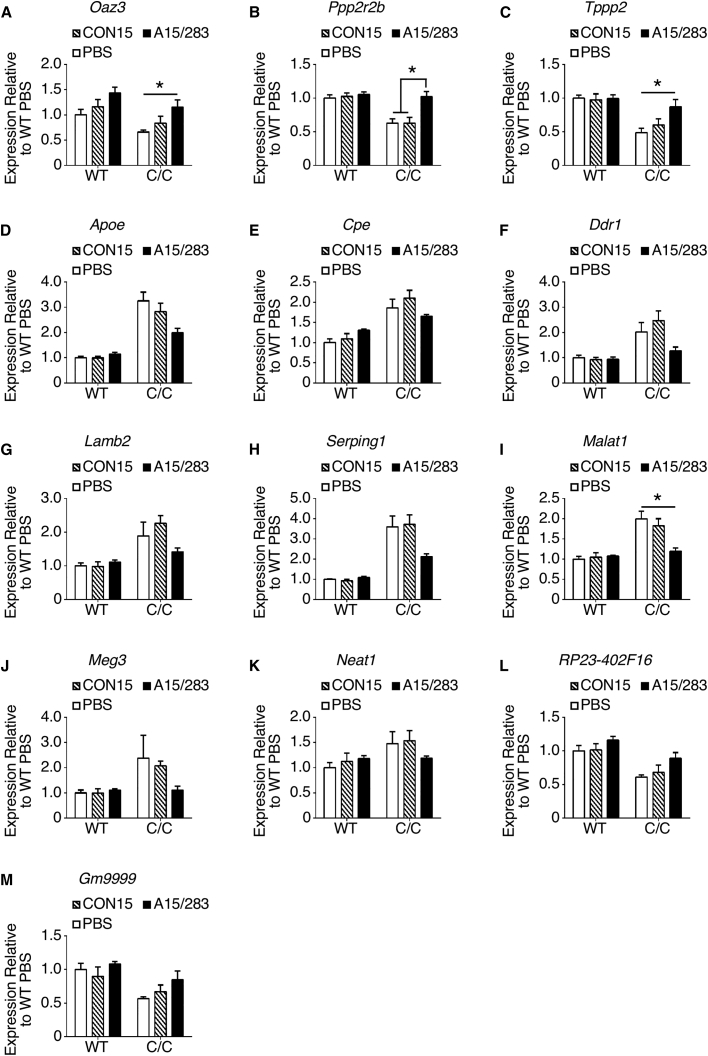
Having shown that an early peripheral increase in SMN leads to improved testicular development and near-normal spermatogenesis in adult C/C testis (Figure 3), we enquired whether SC administration of A15/283 into C/C pups at P1 and P3 could suppress aberrant expression of genes in adult testis. On the basis of the recent RNA-seq analysis of C/C testis,29 we randomly chose eight protein-coding genes, expressions of which are highly perturbed in allele C testis. Three of these chosen genes (Oaz3, Ornithine decarboxylase antizyme 3; Ppp2r2b, Protein phosphatase-2 regulatory subunit B-β; and Tppp2, Tubulin polymerization-promoting protein 2) are highly downregulated in C/C testis; in WT testis, they are all expressed at their peak levels after the appearance of spermatozoa.29 Treatment of C/C males with A15/283 at P1 and P3 nearly restored the expression of Oaz3, Ppp2r2b, and Tppp2 to the WT levels in adult testis examined at P60 (Figures 5A–5C). One-way ANOVA revealed a significant treatment effect for each gene in C/C testis (Oaz3: F(2,11) = 4.619, p = 0.0350; Ppp2r2b: F(2,11) = 7.861, p = 0.0076; Tppp2: F(2,11) = 4.643, p = 0.0345). We next examined five genes (Apoe, Apolipoprotein E; Cpe, Carboxypeptidase E; Ddr1, Discoidin domain receptor 1; Lamb2, Laminin β2; and Serping1, Serine peptidase inhibitor G1) that are highly upregulated in the testis of C/C mice.29 Similar to the downregulated genes (Figures 5A–5C), SC administration of A15/283 into C/C pups at P1 and P3 nearly corrected the expression of the abnormally upregulated genes in P60 adult testis (Figures 5D–5H). One-way ANOVA revealed a near-significant treatment effect for Apoe (F(2,11) = 3.950, p = 0.0509), Ddr1 (F(2,11) = 2.947, p = 0.0944), and Serping1 (F(2,11) = 3.521, p = 0.0658) in C/C testis. Restored expression of these protein-coding genes in adult C/C testis supports that a modest increase in SMN during early testicular development results in the normalization of various signaling pathways and cytoskeletal dynamics in the testis.
Figure 5.
Early Peripheral A15/283 Treatment Partially Normalized Dysregulated Gene Expression in C/C Testis
(A–M) Each panel shows the relative expression of a gene previously shown to be dysregulated in P42 C/C testis.29 Expression for each gene is relative to the WT PBS group (set at 1.0). For WT, n = 6, 7, and 5 mice for PBS, CON15, and A15/283 groups, respectively. For C/C, n = 5, 5, and 4 mice for PBS, CON15, and A15/283 groups, respectively. Error bars on graphs indicate SEM. For each gene, data were analyzed with a two-way ANOVA followed by Tukey’s multiple comparisons test. *p < 0.05 indicates a significant difference between the means.
We also examined the effect of ASO treatment on expression of five long-noncoding RNAs (lncRNAs), specifically Malat1, Meg3, Neat1, RP23-402F16, and Gm9999. These lncRNAs represent a broad spectrum of sequences with consequence to the sequestration of a variety of RNA-binding proteins. lncRNAs are also involved in posttranscriptional regulations through complementary interactions with genomic sequences. Although Malat1, Meg3, and Neat1 are substantially upregulated, RP23-402F16 and Gm9999 are downregulated in adult testis of C/C mice.29 Treatment of C/C pups with A15/283 at P1 and P3 restored the near-normal testicular expression of all five lncRNAs examined at P60 (Figures 5I–5M). Of note, one-way ANOVA revealed a significant treatment effect for Malat1 (F(2,11) = 6.115, p = 0.0164) and a near-significant treatment effect for RP23-402F16 (F(2,11) = 2.882, p = 0.0985) in C/C testis. Overall, our results underscore that a modest increase in testicular SMN caused by SC administration of A15/283 into C/C pups at P1 and P3 restores near-normal expression of both protein-coding genes and lncRNAs in adult testis.
Discussion
Here, we report gender-specific amelioration of the phenotype of mild SMA mice (C/C mice) upon partial restoration of SMN by peripheral administration of an ISS-N2-targeting ASO (A15/283). Owing to its location deep within intron 7 and the mechanism behind its inhibitory effect on exon 7 splicing, ISS-N2 differs from all previously reported antisense targets for splicing correction in SMA.32, 37 Although the significance of an intra-intronic structure was recently implicated in the pathogenesis of Pelizaus-Merzbacher disease,42 this study is the first to validate the therapeutic efficacy of an ASO that targets a similar structure formed by a long-distance intronic interaction, which affects splicing. SC administration of A15/283 into C/C pups at P1 and P3 led to an approximately 40% increase in SMN protein in liver examined at P7; however, the effect of A15/283 on SMN levels was much less evident in other tissues (Figure 1). Nevertheless, we observed a noticeable amelioration of tail necrosis in C/C mice at P60 (Figure 2). Supporting a gender-specific effect, tail necrosis in A15/283-treated C/C females was delayed more prominently compared to C/C males. This benefit was likely due to less severe vascular necrosis in tails of A15/283-treated C/C females as compared to males. Estrogen plays a protective role in the maintenance of vascular endothelial cells.43 Thus, the pronounced delay in tail necrosis onset and near-normal tail vasculature of C/C females treated with A15/283 could be due to the synergistic effect of the protective estrogen signaling and the increased SMN caused by A15/283. The sexual dimorphic response observed in this study clearly underscores the necessity of taking sex into account when considering treatment strategies for SMA.
Our treatment regimen was aimed at modestly increasing SMN protein in peripheral organs. SC administration did not affect SMN2 splicing or SMN protein in the brain, an expected outcome because delivery of ASO is limited by the blood-brain barrier. Upon systemic administration, PS ASOs generally bind plasma proteins44 and accumulate primarily in the liver and kidney.45 A15/283 promoted a more pronounced effect on SMN2 splicing and SMN protein in the liver compared to the kidney (Figure 1). It is possible that the A15/283-protein complex was more effectively endocytosed by the liver compared to the kidney cells to produce the significant effect on SMN2 splicing and SMN protein. Alternatively, A15/283 endocytosed by kidney cells may be degraded and/or excreted before it reaches its cellular target to exert its maximal effect in the kidney. Early A15/283 administration did benefit the C/C phenotype, although it was unable to produce any significant effect on the weight of C/C mice (Figure S4). It is likely that a temporary postnatal elevation of SMN was not sufficient to drastically change the trajectory of BW gain in SMA mice. Supporting this argument, severe SMA mice receiving early postnatal treatments with ISS-N1-targeting ASOs that restore very high levels of SMN could never catch up with the BW of the heterozygous littermates or WT mice.33, 34 Moreover, gain in BW of SMA mice is not a reliable measurement of therapeutic efficacy because gene-therapy-based strategies that provide the sustained production of SMN show different survival benefits, despite attaining a similar high BW upon treatment with the different concentrations of viral vectors.46 We next focused on the novel testis phenotype in the C/C male as another outcome measure to assess the efficacy of the A15/283 treatment. SMN expression in C/C testis is significantly reduced during the early postnatal period, particularly before P12, and drastically increases after P18 due to a testis-specific switch in SMN2 exon 7 splicing.29 Our study design allowed us to assess whether a SMN increase during the early postnatal period would alter the phenotype of the adult testis. The time period before P18 corresponds to maturation of spermatogonia, mitotic division of spermatogonia to spermatocytes, and meiosis I.47 Following a modest increase in SMN at P7 due to SC administration of A15/283 at P1 and P3, adult C/C males displayed a remarkable improvement in testicular development (Figure 3). Consistently, A15/283 treatment minimized perturbations in the expression patterns of apoptotic genes observed in the testis of adult C/C males. However, because our treatment strategy did not restore enough SMN for an extended period of time, A15/283-treated adult C/C testes were still smaller than WT testes and showed a significantly higher percentage of TUNEL-positive tubules. Nevertheless, the treatment of C/C pups with A15/283 at P1 and P3 restored near WT levels of sperm counts measured at P60. These results support that a modest increase in SMN during the early stages of testicular development would likely have a positive impact on overall male reproductive health.
The adult testis of mild SMA mice is unique in maintaining SMN levels comparable to those of WT mice. Still, adult C/C testes display massive perturbations in expressions of several protein-coding genes and lncRNAs.29 These findings suggest that the low level of SMN during early stages of testicular development has a long-term negative consequence on gene expression in testis. The results in this study support the reversible nature of programming of the testicular transcriptome because expressions of several genes in adult C/C testis were restored to near WT levels upon SC administration of A15/283 at P1 and P3. Oaz3, Ppp2r2b, and Tppp2 are three highly downregulated genes in adult C/C testis.29 Oaz3 codes for an ornithine decarboxylase antizyme that regulates polyamine synthesis. However, in testis, Oaz3 is translated into a shortened protein that lacks the antizyme activity and instead interacts with myosin phosphatase targeting subunit 3 to modulate protein phosphatase 1 activity.48, 49 Interestingly, knocking out of Oaz3 in the mouse has no effect on testicular development, but males become infertile due to separation of the sperm head and tail, potentially because of abnormal phosphatase activity.50 Hence, the restored expression of Oaz3 due to A15/283 treatment has a direct consequence on the maintenance of proper sperm morphology. Similarly, the restored expression of Ppp2r2b, which codes for the regulatory subunit of protein phosphatase 2 is likely to correct critical signaling pathways during spermatogenesis. Mutations in Ddr1 that codes for Discoidin domain receptor 1 have been implicated in idiopathic nonobstructive azoospermia.51 The restored expression of Ddr1 in adult C/C males is yet another indication of the long-term positive effect of the early SMN upregulation on the expression of genes critical for spermatogenesis in adult mice. In addition to protein-coding genes, we observed the restored expressions of several upregulated lncRNAs, including Meg3, Malat1, and Neat1, in adult C/C testes caused by treatment with A15/283 at P1 and P3. Meg3 is a tumor suppressor,52 Malat1 regulates phosphorylation of a splicing factor,53 and Neat1 serves as a scaffold for the formation of paraspeckles, in which many RNA-binding proteins are sequestered.54 Our finding that SMN controls expression of several lncRNAs in a time-sensitive and concentration-dependent manner in testis of C/C males supports a wider role of SMN in RNA metabolism in mammalian testis. Future studies will determine how SMN protein levels during the short time window of early testicular development program the transcriptome of the adult testis.
Several studies have demonstrated the anti-apoptotic properties of the SMN protein.55, 56, 57, 58 Low levels of SMN cause DNA fragmentation in muscle and motor neurons of SMA patients and mouse models of the disease.59, 60, 61, 62, 63 Apoptosis is tightly regulated, in part, by proper phosphorylation of pro- and anti-apoptotic factors.64 As discussed above, our gene expression results point to potential abnormal phosphatase activity in the C/C testis, indicated by reduced expression of Ppp2r2b and Oaz3 (Figure 5). The deregulated phosphatase activity could lead to aberrant phosphorylation of apoptotic factors. For example, Bax, a prominent player in mitochondria-mediated apoptosis, directly associates with and is regulated by protein phosphatase 2.65 Increased protein phosphatase 2 activity could increase Bax phosphorylation to facilitate apoptosis initiation, caspase activation, and subsequent DNA damage as detected by TUNEL staining. Bax was previously implicated in motor neuron degeneration in the severe Taiwanese SMA mouse.62 Abnormal phosphatase activity could also disrupt the physiological phosphorylation of other proteins that regulate apoptosis. An early increase in SMN through A15/283 treatment ameliorates, but does not fully prevent apoptosis in the sexually mature C/C testis. Thus, SMN in the testis may help regulate the proper post-translational modification of proteins, including Bax, required for cell survival. Our findings further support a role of SMN in apoptosis regulation and extend this function to the testis.
SMA is a model genetic disease, which could be treated by the restoration of a functional protein through correction of splicing of a single exon.66, 67 The diversity of approaches for achieving this goal provides the alternative therapeutic options for patients with different disease severities. Interestingly, in the case of a recently reported osteoarthritis study, SMN is expressed at an aberrantly high level in cartilage.68 Thus, increasing SMN protein too much could also negatively affect tissue homeostasis and function. This report suggests the need for therapeutic approaches that show high efficacy, even when they upregulate SMN only modestly. The findings reported in this study underscore the utility of a deep intronic structure as a promising therapeutic target for splicing correction. Our results demonstrate for the first time a gender-specific outcome that could be achieved with only moderate and temporary restoration of SMN using a relatively noninvasive procedure. Although we attribute the amelioration of male reproductive phenotype in mild SMA to the increase of SMN in testis during the early postnatal period, we cannot rule out that some of the beneficial effects on testis phenotype could be due to the partial restoration of SMN in other peripheral tissues. One of the notable findings of this study is the near-total correction of the transcriptome profile in adult C/C testis upon a modest postnatal increase of SMN. Now that we have shown the gender-specific benefits of an early intervention in mild SMA mice using A15/283, the stage is set for additional studies employing different administration regimens as well as different mouse models of SMA.
Materials and Methods
Study Design
The objective for this study was to examine the in vivo efficacy of an ASO targeted to a deep intronic structure in SMN2 on the phenotype of a mild mouse model of SMA when administered peripherally during early postnatal development. We chose a sample size of at least seven mice per group by utilizing a power analysis based on the results of a pilot study. In this pilot study, we observed a gender-specific delay of tail necrosis in C/C mice. Subsequently, we initiated a blinded and randomized study to more fully investigate potential gender-specific effects. Before initiating this study, we chose to euthanize all mice at P60 based on observations from our pilot study. At this age, there was a clear difference in tail length between male and female C/C mice treated with A15/283 that would allow us to examine the mechanism behind the gender-specific response to ASO treatment. Additionally, mice are sexually mature at P60, so we could examine the benefit of an early increase in SMN protein on the development of C/C testis. For blinding, each compound (A15/283, CON15, and PBS) was coded with a letter. New litters of pups were randomly assigned one of the coded treatments, and all pups in the litter were injected with the same compound. Thus, the person injecting, observing, and euthanizing the mice was blinded to which treatment was administered. For histological analysis, slides were coded so that the experimenter performing the analysis was unaware of the treatment.
ASOs
RNA ASOs for in vivo injection were synthesized by TriLink Technologies. Each ASO had terminal modifications as previously described to increase distribution and stability in tissues (Figure 1A).38 Sequences for the ASOs were A15/283 (5′-AAG UCU GCU GGU CUG-3′) and CON15 (5′-GGG UCA UAU CUC UGG-3′). CON15 was a scrambled version of A15/283 predicted to have no complementary target in the mouse genome. ASO 261-278, 283-297 and anti-N1 that were used for in vitro studies were obtained from Dharmacon. The sequence for ASO 283-297 was the same as A15/283. The sequence for ASO 261-278 was 5′-СUA GUG AUA UAA AAU GGC- 3′ and the sequence for anti-N1 was 5′-AUU CAC UUU CAU AAU GCU GG-3′.
Cell Culture and ASO Transfection
Primary SMA type I patient fibroblasts were obtained from Coriell Cell Repositories (repository number GM03813). Cells were grown in minimal essential medium (MEM) (catalog number 10370) supplemented with 15% fetal bovine serum and 1X GlutaMAX-I. All cell culture supplies and growth medium were purchased from Life Technologies. Transient transfections of cells were performed using Lipofectamine-2000 (Life Technologies) following the recommendations provided by the manufacturer. GM03813 cells were plated in six-well plates at a density of ∼2 × 105 cells per well. The next day (∼20 hr later), the cells were transfected with 10 nM (final concentration) ASOs. 24 hr after their transfection, GM03813 cells were lysed with TRIzol Reagent (Life Technologies) directly in culture plates, and total RNA was isolated following the manufacturer’s instructions. To generate cDNA, reverse transcription reactions were performed using a SuperScript III Reverse Transcription kit (Thermo Fisher) and an oligo(dT)12-18 primer. 1 μg of total RNA was used per 5 μL of a reverse transcription (RT) reaction. Subsequent PCR amplifications of endogenous SMN were performed in the presence of a trace amount of [α-32P] dATP (3,000 Ci/mmol, PerkinElmer). For PCR, the primers N24 (5′- CCAGATTCTCTTGATGATGCTGATGCTTTGGG-3′) and P2 (5′- GCATGCAAGCTTCCTTTTTTCTTTCCCAACAC-3′) were used. PCR products were resolved on a native 6% polyacrylamide gel, which were then dried and exposed to a storage phosphor screen overnight, scanned using a Fujifilm FLA-5100 phosphorimager, and analyzed using Multi Gauge software (Fujifilm). The results were confirmed by at least two independent experiments.
Mice and ASO Treatment
All animal protocols were approved by the Institutional Animal Care and Use Committee (IACUC) at Iowa State University (ISU), and all procedures conformed to appropriate regulatory guidelines. Neonates were generated from in-house monogamous breeding pairs with parents heterozygous for the C allele (Smn+/C).23 Only litters with five to nine neonates were treated. All treatments were blinded and randomized (see Study Design for details). On the day after birth, P1, each litter was randomly assigned injection with PBS, CON15, or A15/283. Stock solutions of CON15 and A15/283 were maintained at 20 μg/μL. Neonates were weighed and then administered 80 μg/g BW of the appropriate treatment subcutaneously at the back of the neck. The total volume for all injections was 20 μL. Neonates were administered another dose of the same treatment on P3 following the above protocol. Beginning at P1 and every other day until P59, pups were weighed, tail length was measured, and general appearance and health were noted. No adverse reactions were observed in any of the neonates treated for this study. We injected 15 litters with PBS, 17 litters with CON15, and 18 litters with A15/283. There was no significant difference in average litter size for each group (PBS, 6.467 ± 1.187 pups; CON15, 7.118 ± 1.495 pups; A15/283, 7.167 ± 1.043 pups; F(2,47) = 1.531, p = 0.2268 by one-way ANOVA). To examine the effect of A15/283 on SMN2 splicing and SMN protein, we established monogamous breeding pairs with parents heterozygous for the C allele (Smn+/C). Newborn pups were genotyped, and only SmnC/C neonates were treated at P1 and P3 as described above. Pups were observed until P7, at which time tissue was collected as described below.
Tissue Collection
Mice sacrificed at P60 were deeply anesthetized with a combination of 100 mg/kg BW ketamine and 10 mg/kg BW xylazine. Mice were transcardially perfused with PBS (Thermo Fisher), and tissues were removed and placed in fixative or stored at −80°C for future RNA and/or protein isolation. The right testis, half of the seminal vesicle, half of the uterus, and the right ovary were incubated overnight in Modified Davidson’s Fixative (Electron Microscopy Sciences). The tissues were then placed in 70% ethanol until processing, embedding in paraffin, and sectioning by the histopathology core at ISU. Tails from all mice were stored in 10% neutral buffered formalin (Thermo Fisher) after sacrifice. Tails were decalcified using formic acid and subsequently cut at the base and in the middle and submitted for processing, embedding in paraffin, and sectioning by the histopathology core at ISU. P7 pups were deeply anesthetized with isoflurane at P7. The neonates were decapitated, and tissues were collected and frozen at −80°C until RNA isolation.
Histology
Testis, uterus, ovary, and tail cross sections (5 μm) were stained with H&E by the ISU Histopathology Core and subsequently assessed. For each testis, at least 40 seminiferous tubules were analyzed and assessed for the presence of spermatogenesis. Tubules with all stages of spermatogenesis—Sertoli cells, spermatogonia, spermatocytes, spermatids, and spermatozoa—were considered complete, and the percentage of complete tubules for each testis was determined. Other tissue sections (uterus, ovary, and tail) were examined under the microscope to assess any abnormalities. All images were captured with a Nikon Eclipse TE2300U microscope.
Protein Isolation
Ten volumes of radioimmunoprecipitation assay (RIPA) buffer (Boston BioProducts) supplemented with HALT protease and phosphatase inhibitor cocktail (Thermo Fisher) was added to the tissue. Tissue was homogenized on ice using a hand-held homogenizer with a disposable pestle (VWR) for 30 s. Homogenates were incubated on ice for 30 min and then centrifuged at 12,000 rpm for 30 min at 4°C. The supernatant was removed from the pellet, and protein concentration was determined using the MicroBCA protein assay kit (Thermo Fisher). Homogenates were stored at −80°C for future use.
Western Blotting and Densitometry
Protein was diluted in 2x Laemmli sample buffer (Bio-Rad) with 10% β-mercaptoethanol (Sigma-Aldrich), boiled for 5 min, and then centrifuged at maximum speed for 5 min at room temperature. 15 (testis) or 30 (brain, liver, and kidney) μg of protein were loaded onto 12% SDS-PAGE gels and separated. The separated protein was transferred to polyvinylidene fluoride (PVDF) membranes (Bio-Rad) using the TransBlot Turbo system (Bio-Rad). The blots were incubated in 5% non-fat milk (Walmart) prepared in 1X Tris-buffered saline with 0.05% Tween-20 (TBST). Blots were incubated in primary antibody and diluted in 5% non-fat milk prepared in TBST, either overnight at 4°C (SMN) or for 30 min at 37°C (α-tubulin). The primary antibodies were mouse anti-SMN2 clone SMN-KH (Millipore; MABE230) diluted 1:1,000 or mouse anti-α-tubulin (Sigma-Aldrich; T6199) diluted 1:2,000. Blots were washed in 1X TBST and subsequently incubated in secondary antibody diluted in 5% non-fat milk prepared in 1X TBST for 30 min at 37°C. The secondary antibody was goat anti-mouse IgG conjugated to horseradish peroxidase (Jackson ImmunoResearch; 115-035-003) diluted 1:4,000. Blots were then washed in 1X TBST and developed using Clarity ECL Western Blotting Substrate (Bio-Rad) or West Femto Chemiluminescent Substrate (Thermo Fisher) and a digital imager (UVP). ImageJ software was used to determine the mean intensity for the band of interest. For each sample, the mean intensity for SMN was divided by the mean intensity for α-tubulin to obtain the relative intensity value. For each tissue, this value was normalized to the PBS average to obtain the percent of PBS value.
RNA Isolation and Quantitative Real-Time PCR
RNA was isolated from P60 testis and P7 liver, kidney, brain, and testis using TRIzol reagent (Thermo Fisher) following the manufacturer’s protocol. A portion of the isolated RNA from P60 testis was treated with RQ1 DNase (Promega) and subsequently used for reverse transcription. For P60 testis, 1 μg of RNA was reverse transcribed in a 10-μL reaction using the SuperScript III Reverse Transcription kit (Thermo Fisher) with Random Primers (Promega; 0.25 μg per reaction). A portion of the cDNA was diluted 20-fold with RNase-free water for quantitative real-time PCR (real-time qPCR). Real-time qPCR was performed using the Stratagene Mx3005P QPCR System (Agilent Technologies) and the FastStart Universal SYBR Green Master with Rox (Roche). Table S1 lists the primer sequences. Each reaction was performed with ∼10 ng of cDNA. Gapdh was used at the housekeeping gene for normalization. Data were analyzed using the ΔΔCt method, with WT PBS set as the reference condition.
Semiquantitative Radioactive RT-PCR
For neonatal liver, kidney, brain, and testis, 1 μg of RNA was reverse transcribed in a 10-μL reaction using the SuperScript III Reverse Transcription kit with oligo(dT)12-18 (Thermo Fisher). PCR reactions were performed on 1 μL of cDNA template in 20-μL reactions using Taq polymerase (NEB) and supplemented with 0.8 μCi of [α-32P]dATP (3,000 Ci/mmol, 10 mCi/mL) (Perkin-Elmer). For PCR, N24 and P2-2 (5′-CTTCCTTTTTTCTTTCCCAACAC-3′) primers were used. 6 μL of PCR products were resolved on a native 6% polyacrylamide gel, which were then dried and exposed to a storage phosphor screen overnight, scanned using a Fujifilm FLA-5100 phosphorimager, and analyzed using Multi Gauge software (Fujifilm).
Statistical Analysis
All statistical analysis was performed using the GraphPad Prism v6.0 software (GraphPad Software). All data are expressed as mean ± SEM, and p < 0.05 was considered significant. For comparison of tail length during development, a two-way repeated-measures ANOVA was used to determine whether there was a treatment effect, and Tukey’s multiple comparison test was used to determine significant differences at each time point. For SMN2 splicing and SMN protein densitometry, comparison between each group for each tissue was performed using a one-way ANOVA, along with Tukey’s multiple comparisons test. All other statistical comparisons were made using a two-way ANOVA with Tukey’s multiple comparisons test.
Author Contributions
Conceptualization, M.D.H. and R.N.S; Investigation, M.D.H., E.W.O., R.L.A., and N.N.S.; Writing – Original Draft, M.D.H. and R.N.S.; Writing – Review and Editing, M.D.H., E.W.O., N.N.S., and R.N.S.; Funding Acquisition, R.N.S.
Conflicts of Interest
ISS-N2 target (US patent 20150315582) described in this manuscript was discovered in the Singh laboratory at Iowa State University (Ames, IA, USA). Inventors, including R.N.S., N.N.S., and Iowa State University, could potentially benefit from commercial exploitation of ISS-N2 target. The ISS-N1 target (US7838657) mentioned in this manuscript was discovered in the Singh laboratory at UMass Medical School (MA, USA). Spinraza (nusinersen) is the first FDA-approved drug for the treatment of SMA and is based on the ISS-N1 target.36 Inventors, including R.N.S., N.N.S., and UMass Medical School, are currently benefiting from licensing of the ISS-N1 target to IONIS Pharmaceuticals/Biogen, the makers of Spinraza.
Acknowledgments
This study was supported by grants from the National Institutes of Health (R01 NS055925), Iowa Center for Advanced Neurotoxicology (ICAN), and Salsbury Endowment (Iowa State University, Ames, IA, USA) to R.N.S.
Footnotes
Supplemental Information includes Supplemental Materials and Methods, six figures, and one table and can be found with this article online at http://dx.doi.org/10.1016/j.ymthe.2017.03.036.
Supplemental Information
References
- 1.Lefebvre S., Bürglen L., Reboullet S., Clermont O., Burlet P., Viollet L., Benichou B., Cruaud C., Millasseau P., Zeviani M. Identification and characterization of a spinal muscular atrophy-determining gene. Cell. 1995;80:155–165. doi: 10.1016/0092-8674(95)90460-3. [DOI] [PubMed] [Google Scholar]
- 2.Singh R.N., Howell M.D., Ottesen E.W., Singh N.N. Diverse role of survival motor neuron protein. Biochim. Biophys. Acta. 2017;1860:299–315. doi: 10.1016/j.bbagrm.2016.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lorson C.L., Hahnen E., Androphy E.J., Wirth B. A single nucleotide in the SMN gene regulates splicing and is responsible for spinal muscular atrophy. Proc. Natl. Acad. Sci. USA. 1999;96:6307–6311. doi: 10.1073/pnas.96.11.6307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Monani U.R., Lorson C.L., Parsons D.W., Prior T.W., Androphy E.J., Burghes A.H., McPherson J.D. A single nucleotide difference that alters splicing patterns distinguishes the SMA gene SMN1 from the copy gene SMN2. Hum. Mol. Genet. 1999;8:1177–1183. doi: 10.1093/hmg/8.7.1177. [DOI] [PubMed] [Google Scholar]
- 5.Burnett B.G., Muñoz E., Tandon A., Kwon D.Y., Sumner C.J., Fischbeck K.H. Regulation of SMN protein stability. Mol. Cell. Biol. 2009;29:1107–1115. doi: 10.1128/MCB.01262-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cho S., Dreyfuss G. A degron created by SMN2 exon 7 skipping is a principal contributor to spinal muscular atrophy severity. Genes Dev. 2010;24:438–442. doi: 10.1101/gad.1884910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Awano T., Kim J.-K., Monani U.R. Spinal muscular atrophy: journeying from bench to bedside. Neurotherapeutics. 2014;11:786–795. doi: 10.1007/s13311-014-0293-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vitali T., Sossi V., Tiziano F., Zappata S., Giuli A., Paravatou-Petsotas M., Neri G., Brahe C. Detection of the survival motor neuron (SMN) genes by FISH: further evidence for a role for SMN2 in the modulation of disease severity in SMA patients. Hum. Mol. Genet. 1999;8:2525–2532. doi: 10.1093/hmg/8.13.2525. [DOI] [PubMed] [Google Scholar]
- 9.Feldkötter M., Schwarzer V., Wirth R., Wienker T.F., Wirth B. Quantitative analyses of SMN1 and SMN2 based on real-time lightCycler PCR: fast and highly reliable carrier testing and prediction of severity of spinal muscular atrophy. Am. J. Hum. Genet. 2002;70:358–368. doi: 10.1086/338627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mailman M.D., Heinz J.W., Papp A.C., Snyder P.J., Sedra M.S., Wirth B., Burghes A.H., Prior T.W. Molecular analysis of spinal muscular atrophy and modification of the phenotype by SMN2. Genet. Med. 2002;4:20–26. doi: 10.1097/00125817-200201000-00004. [DOI] [PubMed] [Google Scholar]
- 11.Cuscó I., Barceló M.J., Rojas-García R., Illa I., Gámez J., Cervera C., Pou A., Izquierdo G., Baiget M., Tizzano E.F. SMN2 copy number predicts acute or chronic spinal muscular atrophy but does not account for intrafamilial variability in siblings. J. Neurol. 2006;253:21–25. doi: 10.1007/s00415-005-0912-y. [DOI] [PubMed] [Google Scholar]
- 12.Wirth B., Brichta L., Schrank B., Lochmüller H., Blick S., Baasner A., Heller R. Mildly affected patients with spinal muscular atrophy are partially protected by an increased SMN2 copy number. Hum. Genet. 2006;119:422–428. doi: 10.1007/s00439-006-0156-7. [DOI] [PubMed] [Google Scholar]
- 13.Watihayati M.S., Fatemeh H., Marini M., Atif A.B., Zahiruddin W.M., Sasongko T.H., Tang T.H., Zabidi-Hussin Z.A., Nishio H., Zilfalil B.A. Combination of SMN2 copy number and NAIP deletion predicts disease severity in spinal muscular atrophy. Brain Dev. 2009;31:42–45. doi: 10.1016/j.braindev.2008.08.012. [DOI] [PubMed] [Google Scholar]
- 14.Jedrzejowska M., Milewski M., Zimowski J., Borkowska J., Kostera-Pruszczyk A., Sielska D., Jurek M., Hausmanowa-Petrusewicz I. Phenotype modifiers of spinal muscular atrophy: the number of SMN2 gene copies, deletion in the NAIP gene and probably gender influence the course of the disease. Acta Biochim. Pol. 2009;56:103–108. [PubMed] [Google Scholar]
- 15.Elsheikh B., Prior T., Zhang X., Miller R., Kolb S.J., Moore D., Bradley W., Barohn R., Bryan W., Gelinas D. An analysis of disease severity based on SMN2 copy number in adults with spinal muscular atrophy. Muscle Nerve. 2009;40:652–656. doi: 10.1002/mus.21350. [DOI] [PubMed] [Google Scholar]
- 16.Heier C.R., Satta R., Lutz C., DiDonato C.J. Arrhythmia and cardiac defects are a feature of spinal muscular atrophy model mice. Hum. Mol. Genet. 2010;19:3906–3918. doi: 10.1093/hmg/ddq330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shababi M., Habibi J., Yang H.T., Vale S.M., Sewell W.A., Lorson C.L. Cardiac defects contribute to the pathology of spinal muscular atrophy models. Hum. Mol. Genet. 2010;19:4059–4071. doi: 10.1093/hmg/ddq329. [DOI] [PubMed] [Google Scholar]
- 18.Shababi M., Habibi J., Ma L., Glascock J.J., Sowers J.R., Lorson C.L. Partial restoration of cardio-vascular defects in a rescued severe model of spinal muscular atrophy. J. Mol. Cell. Cardiol. 2012;52:1074–1082. doi: 10.1016/j.yjmcc.2012.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Boyer J.G., Ferrier A., Kothary R. More than a bystander: the contributions of intrinsic skeletal muscle defects in motor neuron diseases. Front. Physiol. 2013;4:356. doi: 10.3389/fphys.2013.00356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gombash S.E., Cowley C.J., Fitzgerald J.A., Iyer C.C., Fried D., McGovern V.L., Williams K.C., Burghes A.H., Christofi F.L., Gulbransen B.D. SMN deficiency disrupts gastrointestinal and enteric nervous system function in mice. Hum. Mol. Genet. 2015;24:3847–3860. doi: 10.1093/hmg/ddv127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sintusek P., Catapano F., Angkathunkayul N., Marrosu E., Parson S.H., Morgan J.E., Muntoni F., Zhou H. Histopathological defects in intestine in severe spinal muscular atrophy mice are improved by systemic antisense oligonucleotide treatment. PLoS ONE. 2016;11:e0155032. doi: 10.1371/journal.pone.0155032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vitte J.M., Davoult B., Roblot N., Mayer M., Joshi V., Courageot S., Tronche F., Vadrot J., Moreau M.H., Kemeny F. Deletion of murine Smn exon 7 directed to liver leads to severe defect of liver development associated with iron overload. Am. J. Pathol. 2004;165:1731–1741. doi: 10.1016/S0002-9440(10)63428-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Osborne M., Gomez D., Feng Z., McEwen C., Beltran J., Cirillo K., El-Khodor B., Lin M.Y., Li Y., Knowlton W.M. Characterization of behavioral and neuromuscular junction phenotypes in a novel allelic series of SMA mouse models. Hum. Mol. Genet. 2012;21:4431–4447. doi: 10.1093/hmg/dds285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Szunyogova E., Zhou H., Maxwell G.K., Powis R.A., Francesco M., Gillingwater T.H., Parson S.H. Survival motor neuron (SMN) protein is required for normal mouse liver development. Sci. Rep. 2016;6:34635. doi: 10.1038/srep34635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thomson A.K., Somers E., Powis R.A., Shorrock H.K., Murphy K., Swoboda K.J., Gillingwater T.H., Parson S.H. Survival of motor neurone protein is required for normal postnatal development of the spleen. J. Anat. 2017;230:337–346. doi: 10.1111/joa.12546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Riessland M., Ackermann B., Förster A., Jakubik M., Hauke J., Garbes L., Fritzsche I., Mende Y., Blumcke I., Hahnen E. SAHA ameliorates the SMA phenotype in two mouse models for spinal muscular atrophy. Hum. Mol. Genet. 2010;19:1492–1506. doi: 10.1093/hmg/ddq023. [DOI] [PubMed] [Google Scholar]
- 27.Richert J.R., Antel J.P., Canary J.J., Maxted W.C., Groothuis D. Adult onset spinal muscular atrophy with atrophic testes: report of two cases. J. Neurol. Neurosurg. Psychiatry. 1986;49:606–608. doi: 10.1136/jnnp.49.5.606-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zerres K., Rudnik-Schöneborn S. 93rd ENMC international workshop: non-5q-spinal muscular atrophies (SMA) - clinical picture (6-8 April 2001, Naarden, The Netherlands) Neuromuscul. Disord. 2003;13:179–183. doi: 10.1016/s0960-8966(02)00211-0. [DOI] [PubMed] [Google Scholar]
- 29.Ottesen E.W., Howell M.D., Singh N.N., Seo J., Whitley E.M., Singh R.N. Severe impairment of male reproductive organ development in a low SMN expressing mouse model of spinal muscular atrophy. Sci. Rep. 2016;6:20193. doi: 10.1038/srep20193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Singh N.K., Singh N.N., Androphy E.J., Singh R.N. Splicing of a critical exon of human Survival Motor Neuron is regulated by a unique silencer element located in the last intron. Mol. Cell. Biol. 2006;26:1333–1346. doi: 10.1128/MCB.26.4.1333-1346.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sivanesan S., Howell M.D., Didonato C.J., Singh R.N. Antisense oligonucleotide mediated therapy of spinal muscular atrophy. Transl. Neurosci. 2013;4:1–7. doi: 10.2478/s13380-013-0109-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Singh N.N., Lee B.M., DiDonato C.J., Singh R.N. Mechanistic principles of antisense targets for the treatment of spinal muscular atrophy. Future Med. Chem. 2015;7:1793–1808. doi: 10.4155/fmc.15.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hua Y., Sahashi K., Rigo F., Hung G., Horev G., Bennett C.F., Krainer A.R. Peripheral SMN restoration is essential for long-term rescue of a severe spinal muscular atrophy mouse model. Nature. 2011;478:123–126. doi: 10.1038/nature10485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Porensky P.N., Mitrpant C., McGovern V.L., Bevan A.K., Foust K.D., Kaspar B.K., Wilton S.D., Burghes A.H. A single administration of morpholino antisense oligomer rescues spinal muscular atrophy in mouse. Hum. Mol. Genet. 2012;21:1625–1638. doi: 10.1093/hmg/ddr600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhou H., Janghra N., Mitrpant C., Dickinson R.L., Anthony K., Price L., Eperon I.C., Wilton S.D., Morgan J., Muntoni F. A novel morpholino oligomer targeting ISS-N1 improves rescue of severe spinal muscular atrophy transgenic mice. Hum. Gene Ther. 2013;24:331–342. doi: 10.1089/hum.2012.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ottesen E.W. ISS-N1 makes the first FDA-approved drug for spinal muscular atrophy. Transl. Neurosci. 2017;8:1–6. doi: 10.1515/tnsci-2017-0001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Singh N.N., Lawler M.N., Ottesen E.W., Upreti D., Kaczynski J.R., Singh R.N. An intronic structure enabled by a long-distance interaction serves as a novel target for splicing correction in spinal muscular atrophy. Nucleic Acids Res. 2013;41:8144–8165. doi: 10.1093/nar/gkt609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Keil J.M., Seo J., Howell M.D., Hsu W.H., Singh R.N., DiDonato C.J. A short antisense oligonucleotide ameliorates symptoms of severe mouse models of spinal muscular atrophy. Mol. Ther. Nucleic Acids. 2014;3:e174. doi: 10.1038/mtna.2014.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Goebl N., Berridge B., Wroblewski V.J., Brown-Augsburger P.L. Development of a sensitive and specific in situ hybridization technique for the cellular localization of antisense oligodeoxynucleotide drugs in tissue sections. Toxicol. Pathol. 2007;35:541–548. doi: 10.1080/01926230701338958. [DOI] [PubMed] [Google Scholar]
- 40.Hsieh-Li H.M., Chang J.G., Jong Y.J., Wu M.H., Wang N.M., Tsai C.H., Li H. A mouse model for spinal muscular atrophy. Nat. Genet. 2000;24:66–70. doi: 10.1038/71709. [DOI] [PubMed] [Google Scholar]
- 41.Bogdanik L.P., Osborne M.A., Davis C., Martin W.P., Austin A., Rigo F., Bennett C.F., Lutz C.M. Systemic, postsymptomatic antisense oligonucleotide rescues motor unit maturation delay in a new mouse model for type II/III spinal muscular atrophy. Proc. Natl. Acad. Sci. USA. 2015;112:E5863–E5872. doi: 10.1073/pnas.1509758112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Taube J.R., Sperle K., Banser L., Seeman P., Cavan B.C.V., Garbern J.Y., Hobson G.M. PMD patient mutations reveal a long-distance intronic interaction that regulates PLP1/DM20 alternative splicing. Hum. Mol. Genet. 2014;23:5464–5478. doi: 10.1093/hmg/ddu271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chakrabarti S., Morton J.S., Davidge S.T. Mechanisms of estrogen effects on the endothelium: an overview. Can. J. Cardiol. 2014;30:705–712. doi: 10.1016/j.cjca.2013.08.006. [DOI] [PubMed] [Google Scholar]
- 44.Geary R.S. Antisense oligonucleotide pharmacokinetics and metabolism. Expert Opin. Drug Metab. Toxicol. 2009;5:381–391. doi: 10.1517/17425250902877680. [DOI] [PubMed] [Google Scholar]
- 45.Juliano R.L. The delivery of therapeutic oligonucleotides. Nucleic Acids Res. 2016;44:6518–6548. doi: 10.1093/nar/gkw236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Meyer K., Ferraiuolo L., Schmelzer L., Braun L., McGovern V., Likhite S., Michels O., Govoni A., Fitzgerald J., Morales P. Improving single injection CSF delivery of AAV9-mediated gene therapy for SMA: a dose-response study in mice and nonhuman primates. Mol. Ther. 2015;23:477–487. doi: 10.1038/mt.2014.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bellvé A.R., Cavicchia J.C., Millette C.F., O’Brien D.A., Bhatnagar Y.M., Dym M. Spermatogenic cells of the prepuberal mouse. Isolation and morphological characterization. J. Cell Biol. 1977;74:68–85. doi: 10.1083/jcb.74.1.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fitzgerald C., Sikora C., Lawson V., Dong K., Cheng M., Oko R., van der Hoorn F.A. Mammalian transcription in support of hybrid mRNA and protein synthesis in testis and lung. J. Biol. Chem. 2006;281:38172–38180. doi: 10.1074/jbc.M606010200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ruan Y., Cheng M., Ou Y., Oko R., van der Hoorn F.A. Ornithine decarboxylase antizyme Oaz3 modulates protein phosphatase activity. J. Biol. Chem. 2011;286:29417–29427. doi: 10.1074/jbc.M111.274647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tokuhiro K., Isotani A., Yokota S., Yano Y., Oshio S., Hirose M., Wada M., Fujita K., Ogawa Y., Okabe M. OAZ-t/OAZ3 is essential for rigid connection of sperm tails to heads in mouse. PLoS Genet. 2009;5:e1000712. doi: 10.1371/journal.pgen.1000712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ramasamy R., Ridgeway A., Lipshultz L.I., Lamb D.J. Integrative DNA methylation and gene expression analysis identifies discoidin domain receptor 1 association with idiopathic nonobstructive azoospermia. Fertil. Steril. 2014;102:968–973.e3. doi: 10.1016/j.fertnstert.2014.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhou Y., Zhong Y., Wang Y., Zhang X., Batista D.L., Gejman R., Ansell P.J., Zhao J., Weng C., Klibanski A. Activation of p53 by MEG3 non-coding RNA. J. Biol. Chem. 2007;282:24731–24742. doi: 10.1074/jbc.M702029200. [DOI] [PubMed] [Google Scholar]
- 53.Tripathi V., Ellis J.D., Shen Z., Song D.Y., Pan Q., Watt A.T., Freier S.M., Bennett C.F., Sharma A., Bubulya P.A. The nuclear-retained noncoding RNA MALAT1 regulates alternative splicing by modulating SR splicing factor phosphorylation. Mol. Cell. 2010;39:925–938. doi: 10.1016/j.molcel.2010.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Clemson C.M., Hutchinson J.N., Sara S.A., Ensminger A.W., Fox A.H., Chess A., Lawrence J.B. An architectural role for a nuclear noncoding RNA: NEAT1 RNA is essential for the structure of paraspeckles. Mol. Cell. 2009;33:717–726. doi: 10.1016/j.molcel.2009.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kerr D.A., Nery J.P., Traystman R.J., Chau B.N., Hardwick J.M. Survival motor neuron protein modulates neuron-specific apoptosis. Proc. Natl. Acad. Sci. USA. 2000;97:13312–13317. doi: 10.1073/pnas.230364197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Vyas S., Béchade C., Riveau B., Downward J., Triller A. Involvement of survival motor neuron (SMN) protein in cell death. Hum. Mol. Genet. 2002;11:2751–2764. doi: 10.1093/hmg/11.22.2751. [DOI] [PubMed] [Google Scholar]
- 57.Trülzsch B., Garnett C., Davies K., Wood M. Knockdown of SMN by RNA interference induces apoptosis in differentiated P19 neural stem cells. Brain Res. 2007;1183:1–9. doi: 10.1016/j.brainres.2007.09.025. [DOI] [PubMed] [Google Scholar]
- 58.Parker G.C., Li X., Anguelov R.A., Toth G., Cristescu A., Acsadi G. Survival motor neuron protein regulates apoptosis in an in vitro model of spinal muscular atrophy. Neurotox. Res. 2008;13:39–48. doi: 10.1007/BF03033366. [DOI] [PubMed] [Google Scholar]
- 59.Simic G., Seso-Simic D., Lucassen P.J., Islam A., Krsnik Z., Cviko A., Jelasic D., Barisic N., Winblad B., Kostovic I. Ultrastructural analysis and TUNEL demonstrate motor neuron apoptosis in Werdnig-Hoffmann disease. J. Neuropathol. Exp. Neurol. 2000;59:398–407. doi: 10.1093/jnen/59.5.398. [DOI] [PubMed] [Google Scholar]
- 60.Stathas D., Kalfakis N., Kararizou E., Manta P. Spinal muscular atrophy: DNA fragmentation and immaturity of muscle fibers. Acta Histochem. 2008;110:53–58. doi: 10.1016/j.acthis.2007.06.001. [DOI] [PubMed] [Google Scholar]
- 61.Simic G., Mladinov M., Seso Simic D., Jovanov Milosevic N., Islam A., Pajtak A., Barisic N., Sertic J., Lucassen P.J., Hof P.R. Abnormal motoneuron migration, differentiation, and axon outgrowth in spinal muscular atrophy. Acta Neuropathol. 2008;115:313–326. doi: 10.1007/s00401-007-0327-1. [DOI] [PubMed] [Google Scholar]
- 62.Tsai M.S., Chiu Y.T., Wang S.H., Hsieh-Li H.M., Lian W.C., Li H. Abolishing Bax-dependent apoptosis shows beneficial effects on spinal muscular atrophy model mice. Mol. Ther. 2006;13:1149–1155. doi: 10.1016/j.ymthe.2006.02.008. [DOI] [PubMed] [Google Scholar]
- 63.Fayzullina S., Martin L.J. Skeletal muscle DNA damage precedes spinal motor neuron DNA damage in a mouse model of apinal muscular atrophy (SMA) PLoS ONE. 2014;9:e93329. doi: 10.1371/journal.pone.0093329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Niemi N.M., MacKeigan J.P. Mitochondrial phosphorylation in apoptosis: flipping the death switch. Antioxid. Redox Signal. 2013;19:572–582. doi: 10.1089/ars.2012.4982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Xin M., Deng X. Protein phosphatase 2A enhances the proapoptotic function of Bax through dephosphorylation. J. Biol. Chem. 2006;281:18859–18867. doi: 10.1074/jbc.M512543200. [DOI] [PubMed] [Google Scholar]
- 66.Seo J., Howell M.D., Singh N.N., Singh R.N. Spinal muscular atrophy: an update on therapeutic progress. Biochim. Biophys. Acta. 2013;1832:2180–2190. doi: 10.1016/j.bbadis.2013.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Howell M.D., Singh N.N., Singh R.N. Advances in therapeutic development for spinal muscular atrophy. Future Med. Chem. 2014;6:1081–1099. doi: 10.4155/fmc.14.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Cucchiarini M., Madry H., Terwilliger E.F. Enhanced expression of the central survival of motor neuron (SMN) protein during the pathogenesis of osteoarthritis. J. Cell. Mol. Med. 2014;18:115–124. doi: 10.1111/jcmm.12170. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.