Figure 5. Phenotypic conversion of inflammatory macrophages to a tissue resident phenotype is disrupted in Vitamin A-deficient mice.
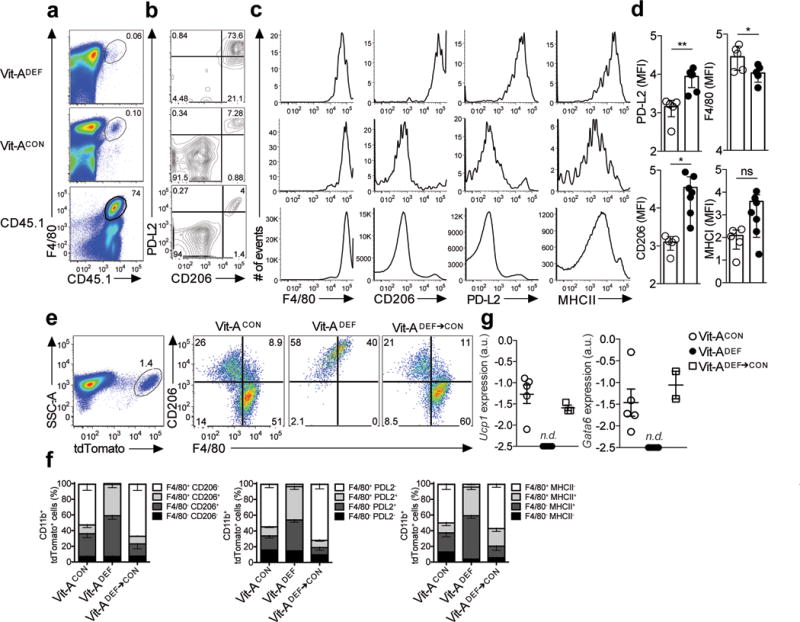
(a) Long-term transfer of monocyte-derived inflammatory macrophages from thioglycollate treated CD45.1 donor mice into CD45.2 recipient vitamin A deficient (Vit-ADEF) mice or control (Vit-ACON) mice, rested for 8 weeks and subsequently treated with IL-4c. Naïve CD45.1 mice were also treated with IL-4c (a) Representative FACS plots of F4/80+ CD45.1+ events and (b) PD-L2 and CD206 expression in CD45.1 cells after 8 weeks post reconstitution in Vit-ACON(n=5) or Vit-ADEF(n=5) mice. (c) Flow cytometry analysis of mean fluorescence intensity for F4/80, CD206, PD-L2 and MHCII in CD45.1 peritoneal macrophages. (d) Quantification of mean fluorescence intensities of F4/80, CD206, PD-L2 and MHCII in CD45.1 macrophages from Vit-ACON(n=5) or Vit-ADEF (n=7) mice. (e) Representative flow cytometry plots of CD206 and F4/80 frequencies in tdTomato+ peritoneal macrophages from Vit-ACON(n=9), Vit-ADEF(n=9) or Vit-ADEF mice switched onto Vit-ACON diet (Vit-ADEF→CON;n=3). (f) Stacked bar graph represents frequencies of F4/80 and CD206 from CD11b+ tdTomato+ cells. (g) RT-PCR analysis of Ucp1 and Gata6 expression in CD11b+ tdTomato+ peritoneal macrophages from (e) normalized to expression of GAPDH. Graphs depict mean ± standard error of the mean of individual mice pooled from 2 independent experiments. Data shown are the mean and s.e.m. ns = not significant, *P<0.05, ***P<0.001 unpaired Students T-test.
