Abstract
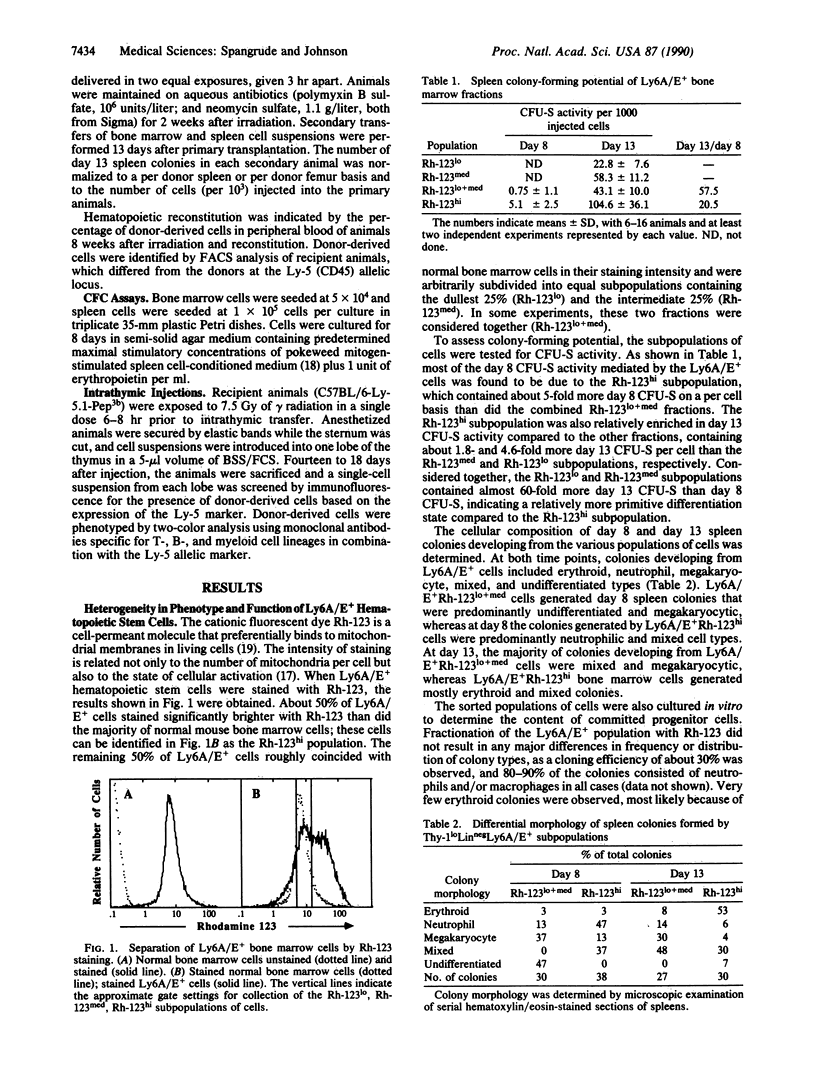
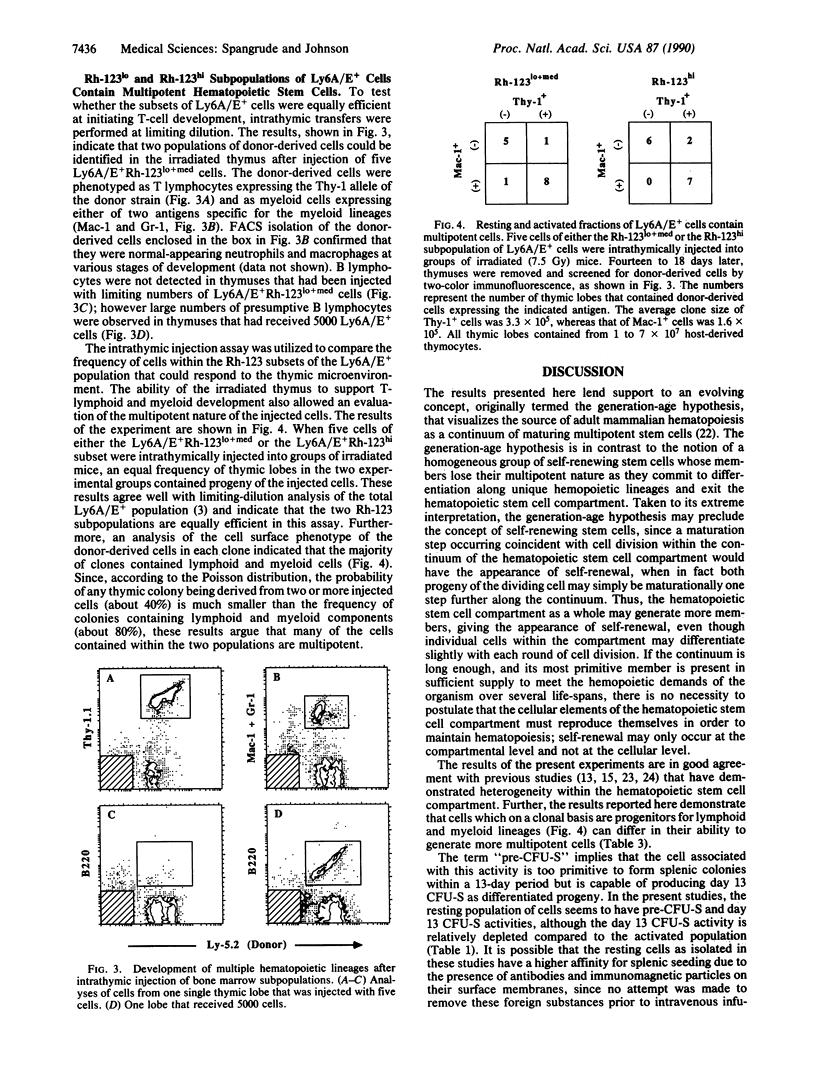
The fluorescent vital dye rhodamine 123 (Rh-123), which preferentially accumulates in mitochondrial membranes, can be used as a probe to indicate mitochondrial and hence cellular activity. In this study, mouse bone marrow hematopoietic stem cells were subdivided into Rh-123lo, Rh-123med, and Rh-123hi populations. The Rh-123lo (resting) population was significantly enriched in cells with a higher proliferative potential compared to the Rh-123hi (activated) population. The resting population exhibited a 20-fold greater ability to differentiate into splenic colony-forming units (CFU-S) relative to the activated population, whereas the activated population contained about 4-fold more day 13 CFU-S on primary transfer relative to the resting population. The two populations produced morphologically distinct splenic colonies; however, the frequency and morphology of in vitro colonies were very similar. Only the resting population provided sufficient stem cells to transfer long-term hematopoietic repopulation to secondary recipient animals after lethal irradiation. On a single cell level, the resting and activated populations exhibited an equivalent ability to differentiate into lymphoid and myeloid progeny. These observations provide further insight into the heterogeneous nature of CFU-S and directly demonstrate that multipotent hematopoietic stem cells are heterogeneous with regard to their clonogenic capacities.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bartelmez S. H., Bradley T. R., Bertoncello I., Mochizuki D. Y., Tushinski R. J., Stanley E. R., Hapel A. J., Young I. G., Kriegler A. B., Hodgson G. S. Interleukin 1 plus interleukin 3 plus colony-stimulating factor 1 are essential for clonal proliferation of primitive myeloid bone marrow cells. Exp Hematol. 1989 Mar;17(3):240–245. [PubMed] [Google Scholar]
- Bertoncello I., Hodgson G. S., Bradley T. R. Multiparameter analysis of transplantable hemopoietic stem cells. II. Stem cells of long-term bone marrow-reconstituted recipients. Exp Hematol. 1988 May;16(4):245–249. [PubMed] [Google Scholar]
- Bertoncello I., Hodgson G. S., Bradley T. R. Multiparameter analysis of transplantable hemopoietic stem cells: I. The separation and enrichment of stem cells homing to marrow and spleen on the basis of rhodamine-123 fluorescence. Exp Hematol. 1985 Nov;13(10):999–1006. [PubMed] [Google Scholar]
- Dexter T. M., Lajtha L. G. Proliferation of haemopoietic stem cells in vitro. Br J Haematol. 1974 Dec;28(4):525–530. doi: 10.1111/j.1365-2141.1974.tb06671.x. [DOI] [PubMed] [Google Scholar]
- Hellman S., Botnick L. E., Hannon E. C., Vigneulle R. M. Proliferative capacity of murine hematopoietic stem cells. Proc Natl Acad Sci U S A. 1978 Jan;75(1):490–494. doi: 10.1073/pnas.75.1.490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgson G. S., Bradley T. R. Properties of haematopoietic stem cells surviving 5-fluorouracil treatment: evidence for a pre-CFU-S cell? Nature. 1979 Oct 4;281(5730):381–382. doi: 10.1038/281381a0. [DOI] [PubMed] [Google Scholar]
- Hunt P., Robertson D., Weiss D., Rennick D., Lee F., Witte O. N. A single bone marrow-derived stromal cell type supports the in vitro growth of early lymphoid and myeloid cells. Cell. 1987 Mar 27;48(6):997–1007. doi: 10.1016/0092-8674(87)90708-2. [DOI] [PubMed] [Google Scholar]
- Inoue T., Bullis J. E., Cronkite E. P., Kubo S. Stem cell proliferation and 125IdUrd incorporation into spleen and whole skeletal tissue. Ann N Y Acad Sci. 1985;459:162–178. doi: 10.1111/j.1749-6632.1985.tb20824.x. [DOI] [PubMed] [Google Scholar]
- Johnson L. V., Walsh M. L., Bockus B. J., Chen L. B. Monitoring of relative mitochondrial membrane potential in living cells by fluorescence microscopy. J Cell Biol. 1981 Mar;88(3):526–535. doi: 10.1083/jcb.88.3.526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson L. V., Walsh M. L., Chen L. B. Localization of mitochondria in living cells with rhodamine 123. Proc Natl Acad Sci U S A. 1980 Feb;77(2):990–994. doi: 10.1073/pnas.77.2.990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung L. C., Johnson G. R. In vitro maintenance of hemopoietic stem cells with lymphoid and myeloid repopulating ability by a cloned murine adherent bone marrow cell line. Exp Hematol. 1987 Oct;15(9):989–994. [PubMed] [Google Scholar]
- Lord B. I., Molineux G., Schofield R., Humphreys E. R., Stones V. A. On the late seeding of CFU-S to the spleen: 8- vs 12-day CFU-S. Exp Hematol. 1989 Aug;17(7):836–842. [PubMed] [Google Scholar]
- Magli M. C., Iscove N. N., Odartchenko N. Transient nature of early haematopoietic spleen colonies. Nature. 1982 Feb 11;295(5849):527–529. doi: 10.1038/295527a0. [DOI] [PubMed] [Google Scholar]
- Metcalf D., Johnson G. R. Production by spleen and lymph node cells of conditioned medium with erythroid and other hemopoietic colony-stimulating activity. J Cell Physiol. 1978 Jul;96(1):31–42. doi: 10.1002/jcp.1040960105. [DOI] [PubMed] [Google Scholar]
- Metcalf D. The molecular control of cell division, differentiation commitment and maturation in haemopoietic cells. Nature. 1989 May 4;339(6219):27–30. doi: 10.1038/339027a0. [DOI] [PubMed] [Google Scholar]
- Müller-Sieburg C. E., Townsend K., Weissman I. L., Rennick D. Proliferation and differentiation of highly enriched mouse hematopoietic stem cells and progenitor cells in response to defined growth factors. J Exp Med. 1988 Jun 1;167(6):1825–1840. doi: 10.1084/jem.167.6.1825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicola N. A., Burgess A. W., Staber F. G., Johnson G. R., Metcalf D., Battye F. L. Differential expression of lectin receptors during hemopoietic differentiation: enrichment for granulocyte-macrophage progenitor cells. J Cell Physiol. 1980 May;103(2):217–237. doi: 10.1002/jcp.1041030207. [DOI] [PubMed] [Google Scholar]
- Ploemacher R. E., Brons N. H. Cells with marrow and spleen repopulating ability and forming spleen colonies on day 16, 12, and 8 are sequentially ordered on the basis of increasing rhodamine 123 retention. J Cell Physiol. 1988 Sep;136(3):531–536. doi: 10.1002/jcp.1041360320. [DOI] [PubMed] [Google Scholar]
- Ploemacher R. E., Brons N. H. In vivo proliferative and differential properties of murine bone marrow cells separated on the basis of rhodamine-123 retention. Exp Hematol. 1988 Dec;16(11):903–907. [PubMed] [Google Scholar]
- Ploemacher R. E., Brons R. H. Separation of CFU-S from primitive cells responsible for reconstitution of the bone marrow hemopoietic stem cell compartment following irradiation: evidence for a pre-CFU-S cell. Exp Hematol. 1989 Mar;17(3):263–266. [PubMed] [Google Scholar]
- Rosendaal M., Hodgson G. S., Bradley T. R. Haemopoietic stem cells are organised for use on the basis of their generation-age. Nature. 1976 Nov 4;264(5581):68–69. doi: 10.1038/264068a0. [DOI] [PubMed] [Google Scholar]
- Rosendaal M., Hodgson G. S., Bradley T. R. Organization of haemopoietic stem cells: the generation-age hypothesis. Cell Tissue Kinet. 1979 Jan;12(1):17–29. doi: 10.1111/j.1365-2184.1979.tb00110.x. [DOI] [PubMed] [Google Scholar]
- Spangrude G. J., Heimfeld S., Weissman I. L. Purification and characterization of mouse hematopoietic stem cells. Science. 1988 Jul 1;241(4861):58–62. doi: 10.1126/science.2898810. [DOI] [PubMed] [Google Scholar]
- Van Zant G. Studies of hematopoietic stem cells spared by 5-fluorouracil. J Exp Med. 1984 Mar 1;159(3):679–690. doi: 10.1084/jem.159.3.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visser J. W., Bauman J. G., Mulder A. H., Eliason J. F., de Leeuw A. M. Isolation of murine pluripotent hemopoietic stem cells. J Exp Med. 1984 Jun 1;159(6):1576–1590. doi: 10.1084/jem.159.6.1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitlock C. A., Tidmarsh G. F., Muller-Sieburg C., Weissman I. L. Bone marrow stromal cell lines with lymphopoietic activity express high levels of a pre-B neoplasia-associated molecule. Cell. 1987 Mar 27;48(6):1009–1021. doi: 10.1016/0092-8674(87)90709-4. [DOI] [PubMed] [Google Scholar]
- van de Rijn M., Heimfeld S., Spangrude G. J., Weissman I. L. Mouse hematopoietic stem-cell antigen Sca-1 is a member of the Ly-6 antigen family. Proc Natl Acad Sci U S A. 1989 Jun;86(12):4634–4638. doi: 10.1073/pnas.86.12.4634. [DOI] [PMC free article] [PubMed] [Google Scholar]




